refer to ‘Chapter 4 Preparation of Medical Devices’.
The sterilizer consistently guarantees a Sterility Assurance Level (SAL) of 10
-6
as defined by the US
Food and Drug Administration (FDA) and International Standards, provided that the materials and
geometrical requirements in accordance with the instructions in this user manual are met.
The pre-validation has shown that this sterilizer can reach a SAL of 10
-6
, including lumens, under
worst-case conditions. For additional technical information related to validation, please contact
Plasmapp or your local Plasmapp Customer Support Representative.
3.3. Sterilization cycle
The STERLINK mini sterilizer is designed to be operated in chamber modes, which is a combined
cycle of the Smart Ready (SR™), Sterilization and Smart Complete (SC™) process. The sterilizer
is designed to operate only with the sterilant cassettes of STERLOAD™
mini. Each cassette has
individual barcode which will be scanned by the sterilizer to start the sterilization cycle
automatically according to the barcode identifying of the cassettes. This automatic scan step reduces
the user error and reliability of sterilization.
The optimized heating and drying process is provided in the SR™ process, during which the
pressure of the vaporizer and chamber is independently controlled, to obtain the venting and
pumping pressure curves.
The sterilization process consists of the two consecutive identical sterilization phases, and the
critical process parameters are controlled to be identical. The validation of the sterilization process
is performed by using the half-cycle overkill method to demonstrate the 10
-6
SAL. In the SC™
process the sterilizing agent is removed before the sterilized medical devices can be removed from
the chamber. To ensure user safety, an independent pressure sensor measures the residual sterilant
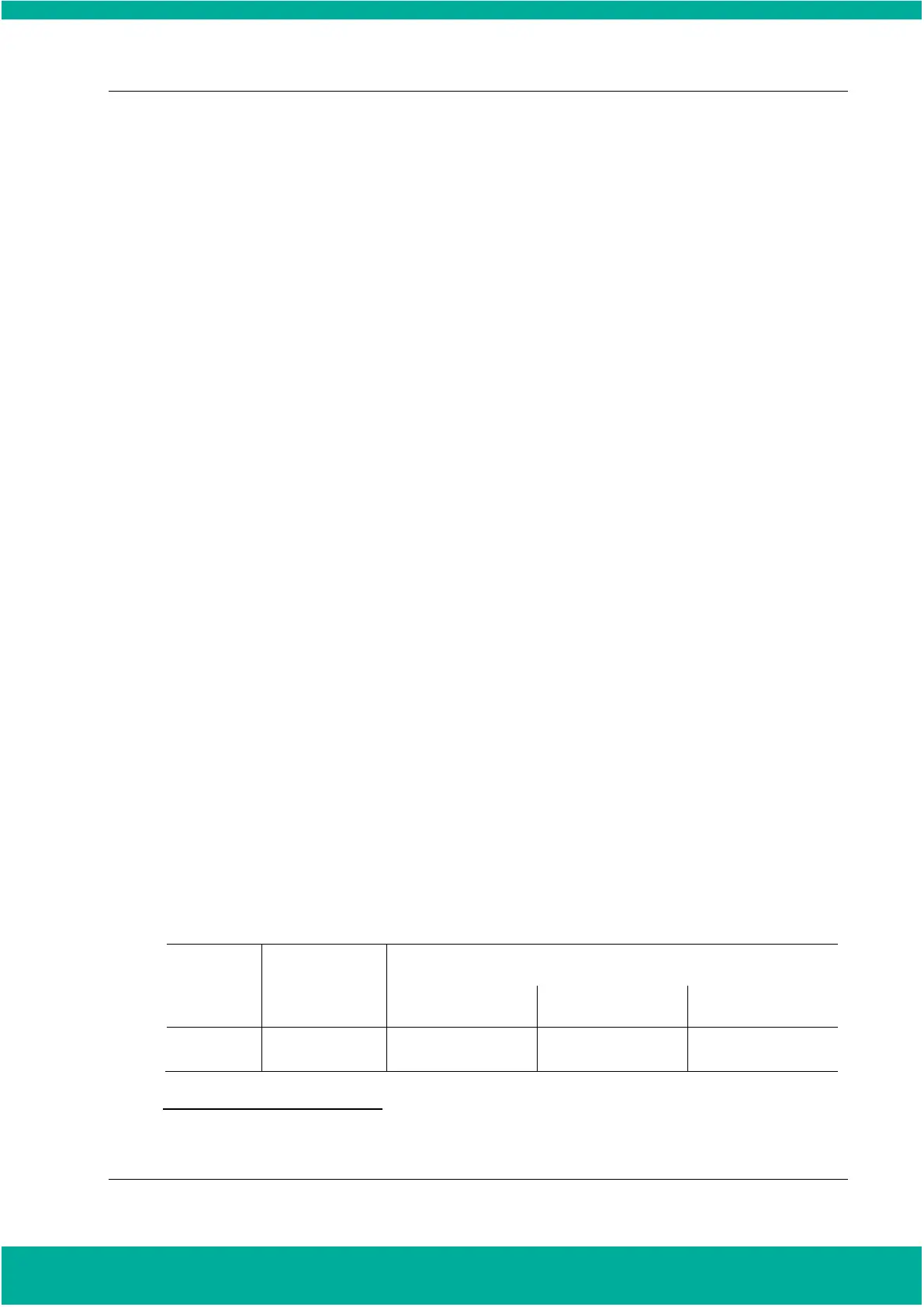
and an optimized completion process is performed. The following table provides a brief description
for sterilization cycle mode.
Sterilization cycle time for CHAMBER mode
 Loading...
Loading...