3
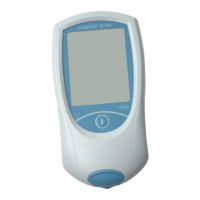
The CoaguChek XS Plus System
The CoaguChek XS Plus System is used to monitor coagulation (blood-clotting) values
(prothrombin time, PT, Quick value) with CoaguChek XS PT test strips.
Please note: This manual contains the information you need to operate and care for
the CoaguChek XS Plus System. Please read this entire manual carefully before you use
the meter.
CLIA Waived
This is a CLIA Waived test system. A Certificate of CLIA Waiver (or higher) is required to
perform the test. Information on obtaining CLIA certificates can be found at
www.cms.hhs.gov/clia.
Before testing, refer to the appropriate package insert for more complete information.
Laboratories with a certificate of waiver must follow the manufacturer’s instructions for
performing the test. 42 CFR 493.15(e)(1).
Any modifications and/or failure to follow test system instructions, including those for
limitations/intended use and performance of QC testing as a failure alert mechanism, results
in use that is considered high complexity and subject to all applicable CLIA requirements.
All applicable state and local laws must be met.
Any adverse reactions experienced with the use of this product, and/or quality problems
should also be reported to the FDA’s MedWatch Adverse Event Reporting program online
(at www.fda.gov/MedWatch/report.htm), by phone (1-800-FDA-1088), or by returning the
postage-paid FDA form 3500 (which may be downloaded from
www.fda.gov/MedWatch/getforms.htm) by mail to (MedWatch, 5600 Fishers Lane, Rockville,
MD 20852-9787) or fax (1-800-FDA-1078).
If there are any problems with the CoaguChek XS Plus meter, notify CMS at
http://www.cms.hhs.gov/clia/ro-map.asp
The CoaguChek XS Plus System
 Loading...
Loading...