Roche Diagnostics
Urisys 1100® · ≥5.7 · Operator's Manual · 9.0
8 1. Introduction
Combur10Test
®
UX test strips are marketed in Canada as
Chemstrip 10 A test strips.
Approvals
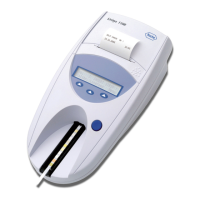
The Urisys 1100
®
system meets the requirements laid
down in:
Regulation (EU) 2017/746 of the European Parliament and
of the Council of 5 April 2017 on in vitro diagnostic
medical devices and repealing Directive 98/79/EC and
Commission Decision 2010/227/EU.
Compliance with the applicable regulation and
directive(s) is provided by means of the Declaration of
Conformity.
!
Incident reporting
r Inform your Roche representative and your local
competent authority about any serious incidents
which may occur when using this product.
Using the Urisys 1100
®
system eliminates factors known
to affect visual evaluation of urine test strips, such as:
• Variable lighting conditions at the workplace
• People’s individual skill at matching colors and their
limits of concentration
• Different reaction times for the test strips
• Clerical errors
• Strong color of the urine sample
Restriction of hazardous substances
The Urisys 1100
®
system (serial# 09652551
and higher) meets the requirements laid
down in: Directive 2011/65/EU of the
European Parliament and of the Council of 8
June 2011 on the restriction of the use of
certain hazardous substances in electrical
and electronic equipment.
BIOHAZARD:
Treat all samples of human origin as being
potentially infectious. Always observe good
laboratory practice.
 Loading...
Loading...