Roche Diagnostics
Urisys 1100® · ≥5.7 · Operator's Manual · 9.0
1. Introduction 7
1. Introduction
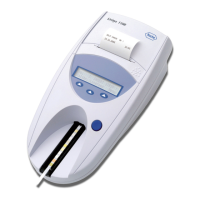
Intended use The Urisys 1100 system is a reflectance photometer
designed to read and evaluate the urine test strips
Combur
10
Test
®
UX from Roche Diagnostics. It reads the
strips under standardized conditions, saves the results to
memory and outputs them via its own inbuilt printer
and/or serial interface.
The Urisys 1100 system is designed for In Vitro Diagnostic
(IVD) use by qualified physicians and laboratory staff.
Not for self-testing.
The instrument is intended for near-patient testing.
Usage environment The Urisys 1100 urine analyzer can be used in non-critical
healthcare environments such as hospital wards and
general practitioner's offices.
User Training The operation of the Urisys 1100 urine analyzer and the
execution of the Combur
10
Test UX is described in the
Urisys 1100 urine analyzer Operator’s Manual. No
dedicated training is required.
Copyright © 2021, Roche Diagnostics GmbH. All rights reserved.
Trademarks COBAS, URISYS, URISYS 1100, COMBUR-TEST,
CHEMSTRIP and REFLOTRON are trademarks of Roche.
Manual version Software version Revision date Amendments
4.0 5.x 2008-06 Operator ID, limited lock-out function,Device
ID, compatibility of barcode reader, ASTM
protocol
5.0 5.x 2013-10 Cobas brand
6.0 5.x 2018-01 New material numbers
Changed test strip handling
Remove of the intended use of
Combur7Test
®
and Combur5Test
®
on
Urisys 1100
®
system.
7.0 ≥ 5.7 2019-03
Combur5Test
®
and Combur7Test
®
removed
from software flow chart.
8.0 ≥ 5.7 2020-06 Quality control (QC) section added.
Test strip tray lifetime added.
Cleaning and maintenance section improved.
Paragraph “Values obtained are implausible
when compared with those from visual
evaluation” revised in section “Error
messages and troubleshooting”.
Paragraph „Control values fall outside the
designated ranges“ added in section “Error
messages and troubleshooting”.
9.0 ≥ 5.7 2021-11 IVDR compliance
y Revision history
 Loading...
Loading...