Wavelength selection C-13
Wavelength selection for chromophore detection
Certain functional groups found in most compounds absorb light selectively.
These groups, known as chromophores, and their behavior can be used to
categorize the detection of sample molecules.
The table below
lists some common chromophores, and their detection
wavelengths (
λ
max
), as well as the molar absorptivity (ε
max
) of each group. Use
this information as a guide to select the optimal operating wavelength for a
particular analysis. Because of the diversity possible within a given sample,
scanning over a range of wavelengths may be necessary to determine the best
wavelength for a particular analysis.
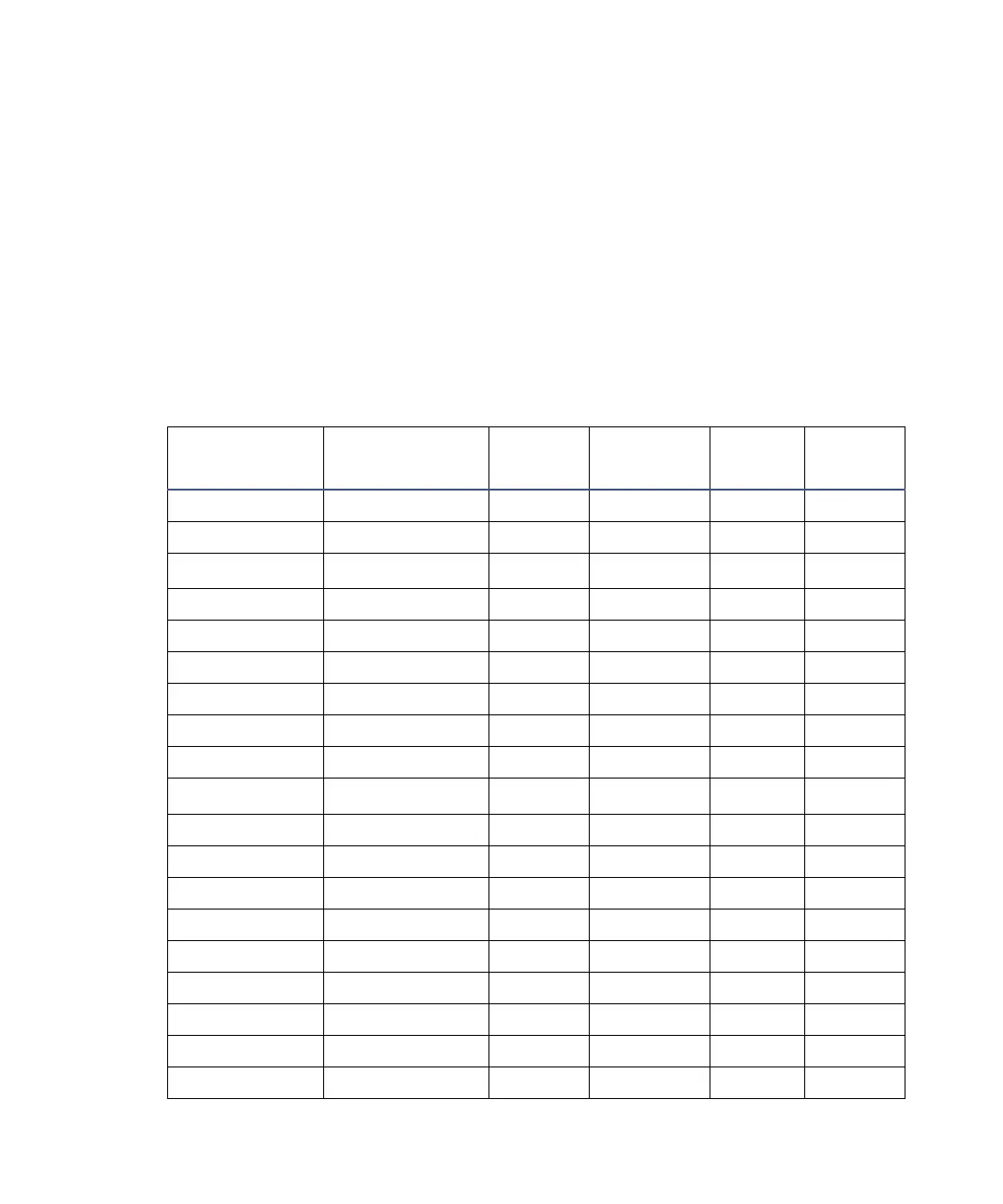
Electronic absorption bands of representative chromophores *
Chromophore
Chemical
Configuration
λ
max
(nm)
∈
max
(L/m/cm)
λ
max
(nm)
∈
max
(L/m/cm)
Ether —O— 185 1000
Thioether —S— 194 4600 215 1600
Amine —NH
2
195 2800
Thiol —SH 195 1400
Disulfide —S—S— 194 5500 255 400
Bromide —Br 208 300
Iodide —I 260 400
Nitrile —C≡N 160 —
Acetylide —C≡C— 175-180 6000
Sulfone —SO
2
— 180 —
Oxime —NOH 190 5000
Azido >C=N— 190 5000
Ethylene —C=C— 190 8000
Ketone >C=O 195 1000 270-285 18-30
Thioketone >C=S 205 strong
Esters —COOR 205 50
Aldehyde —CHO 210 strong 280-300 11-18
Carboxyl —COOH 200-210 50-70
Sulfoxide >S—O 210 1500
 Loading...
Loading...