C-14 Solvent Considerations
*Willard, H. H. and others. Instrumental Methods of Analysis, 6th ed. Litton Educational Publishing,
Inc., 1981. Reprinted by permission of Wadsworth Publishing Co., Belmont, California, 94002.
Mobile phase absorbance
This section lists the absorbances at several wavelengths for frequently used
mobile phases. Choose the mobile phase carefully to reduce baseline noise.
The best mobile phase for your application is one that is transparent at the
chosen detection wavelengths. With such a mobile phase, ensure that any
Nitro —NO
2
210 strong
Nitrile —ONO 220-230 1000-2000 300-400 10
Azo —N=N— 285-400 3-25
Nitroso —N=O 302 100
Nitrate —ONO
2
270
(shoulde
r)
12
Allene —(C=C)
2
—
(acyclic)
210-230 21,000
Allene —(C=C)
3
— 260 35,000
Allene —(C=C)
4
— 300 52,000
Allene —(C=C)
5
— 330 118,000
Allene —(C=C)
2
—
(alicyclic)
230-260 3000-8000
Ethylenic/
Acetylenic
C=C—C≡C 219 6,500
Ethylenic/
Amido
C=C—C=N 220 23,000
Ethylenic/
Carbonyl
C=C—C=O 210-250 10,000-
20,000
Ethylenic/
Nitro
C=C—NO
2
229 9,500
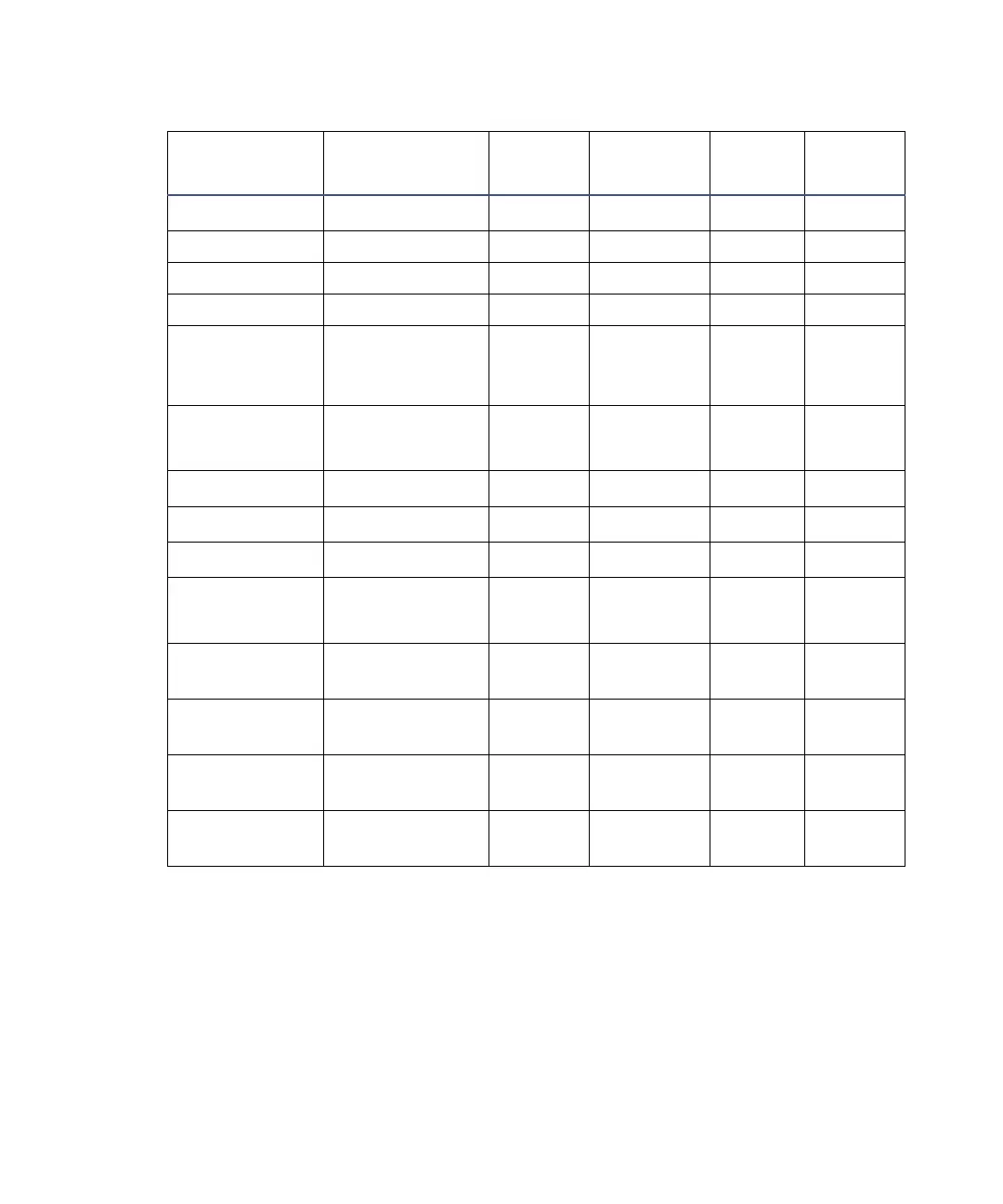
Electronic absorption bands of representative chromophores (Continued)*
Chromophore
Chemical
Configuration
λ
max
(nm)
∈
max
(L/m/cm)
λ
max
(nm)
∈
max
(L/m/cm)
 Loading...
Loading...