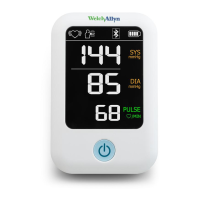
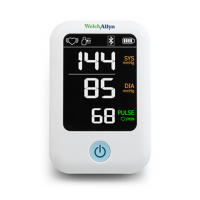
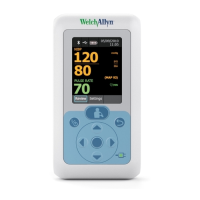
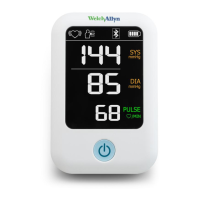
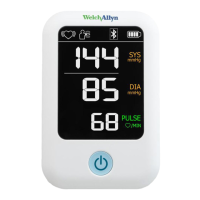
3
Table of contents
Symbols 4
Introduction 5
Preliminary note 5
About these instructions for use 5
Clinical data 5
CE Mark 6
Content 6
Instructions for use 7
Intended use 7
Indications for use 7
Contraindications 7
Essential Performance 7
Side effects of 24-hour blood pressure monitoring 8
Product description 9
Introduction 9
The ABPM 7100 9
Technical Data and environmental conditions 13
Accessories 14
Preparing the ABPM 7100 15
Safety instructions 15
Inserting the batteries 16
Activating the device 18
Setting the time / date 19
Clearing the memory 19
Transferring patient data 19
Setting measurement logs 20
Selecting a suitable cuff 21
Applying the ABP Monitor and cuff 22
Connecting the cuff tubing to the ABPM 7100 24
Positioning the patient for measurement 24
Measurement process 25
Safety instructions 25
Initial measurement 27
24-hour measurement 28
Performing a measurement 28
Cancelling a measurement 28
Unsuccessful measurement 28
Care and Maintenance 29
Cleaning 29
Disinfection 30
Maintenance plan 31
Troubleshooting 32
Basic error sources 32
Transmission error 32
Checklist 32
Error codes 33
Limited Warranty 36
Service Policy 37
EMC Guidelines and Manufacturer Declaration 38
Patient Information - operation of the ABPM 7100 41
WAR
NING The warning statement
identifies an immediate threat. Non-
adherence may lead to the most severe
injuries and to death
CAUTION The caution statement
identifies a possible hazard. Non-
adherence may lead to minor or
moderate injuries
Attention The attention statement marks possible
material damage. Non-adherence may
lead to damage to the device or its
accessories
Note The note statement marks further
information on the ABPM 7100 or its
accessories
INT
ERNAL REFERENCE Marks
references within the document to further
information
EXTERNAL REFERENCE Marks
references to external documents
containing further optional information
Man
datory – Consult Instructions for use Meets essential requirements of
European Medical Device Directive
93/42/EEC
Con
sult Instructions for use, Electronic
version available at Welchallyn.com, or
Hard copy DFU available from Welch
Allyn within 7 days.
B
a
t
tery symbol indicates the type of
power supply
Shipping, storing and environment symbols
Sep
arate the device from other
disposables for recycling.
See www.welchallyn.com/weee
Man
ufacturer
yyyy-mm
Date of manufacture
Ref
erence/Model number Serial number
Reo
rder/Catalog number Batch code
Glo
bal Trade Item Number Protection class
NRTL-Certification
D
e
f
ibrillation-proof type BF applied part MR Unsafe Poses unacceptable risks
to the patient, medical staff or other
persons within the MR (magnetic
resonance) environment

 Loading...
Loading...