1260 Infinity Binary LC - System User Guide 29
Introduction
2
Theory of Using Smaller Particles in Liquid Chromatography
The Theory
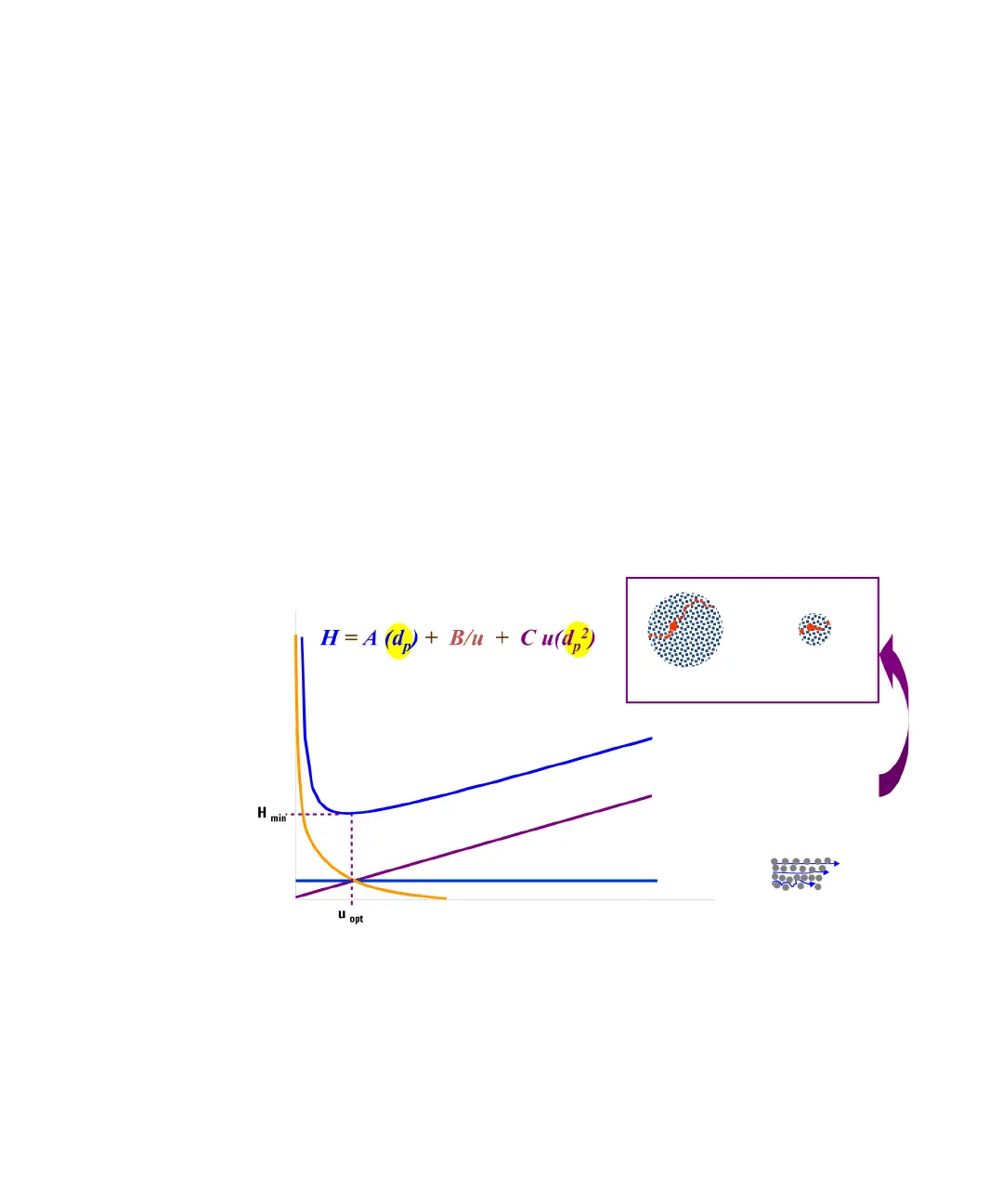
Separation efficiency in HPLC can be described by the van Deemter
equation (Figure 6 on page 29). This results from the plate- height model
that is used to measure the dispersion of analytes as they move down the
column. H is the Height Equivalent to a Theoretical Plate (sometimes
HETP), d
p
is the particle size of the column packing material, u
0
is the
linear velocity of the mobile phase and A, B and C are constants that are
related to the different dispersive forces. The A term relates to eddy
diffusion or multiple flow paths through the column; B relates to
molecular diffusion along the column axis (longitudinal); C relates to mass
transfer of the analyte between the mobile and stationary phases. The
separation is at its most efficient when H is at a minimum. The effect of
each individual term and the combined equation are shown in Figure 6 on
page 29where the plate height is plotted against the linear flow rate
through the column. This type of plot is known as a Van Deemter Curve
and is used to determine the optimum flow rate (minimum point of the
curve) for best efficiency of separation for a column.
Figure 6 A hypothetical Van Deemter curve
GZhjai^c\KVc"9ZZbiZgXjgkZ
GZh^hiVcXZidBVhhIgVch[Zg
Bjai^eVi]IZgb!
:YYn9^[[jh^dc
A^cZVg[adlj
aVg\ZeVgi^XaZ
hbVaaeVgi^XaZ
I]ZdgZi^XVaEaViZ=Z^\]i=
Adc\^ijY^cVaY^[[jh^dc
 Loading...
Loading...











