System Overview | 2-3
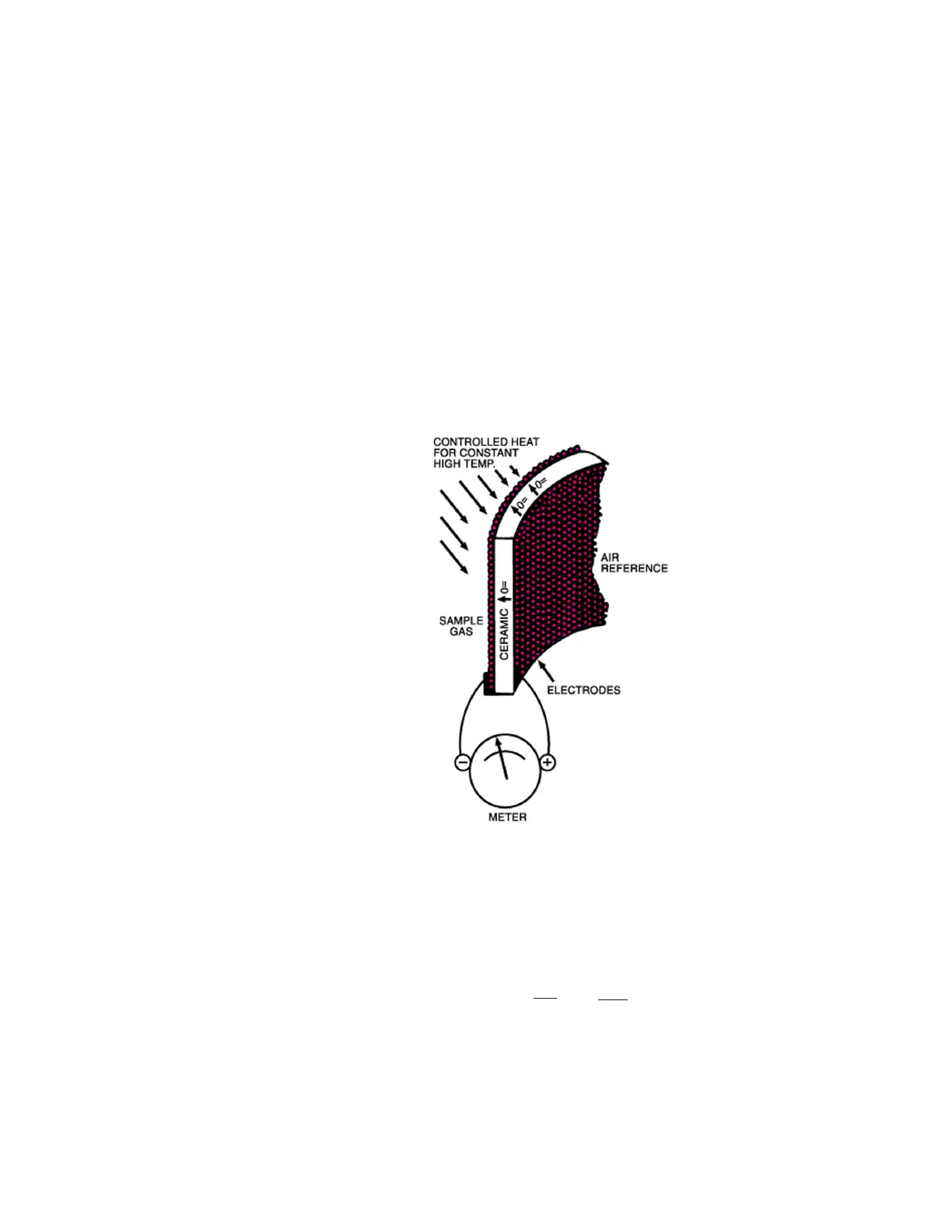
The Oxygen Measuring Cell
The sensing element itself is a closed-end tube made from ceramic zirconi-
um oxide stabilized with an oxide of yttrium or calcium. Porous platinum
coatings on the inside and outside serve as a catalyst and as electrodes.
At high temperatures (generally above 1200 °F/650 °C), oxygen molecules
coming in contact with the platinum electrodes near the sensor become
ionic. As long as the oxygen partial pressures on either side of the cell are
equal, the movement is random and no net flow of ions occurs. If, how-
ever, gases having different oxygen partial pressures are on either side of
the cell, a potentiometric voltage is produced (See Figure 2-1). The mag-
nitude of this voltage is a function of the ratio of the two oxygen partial
pressures. If the oxygen partial pressure of one gas is known, the voltage
produced by the cell indicates the oxygen content of the other gas. A ref-
erence gas, usually air (20.9% O
2
), is used for one of the gases.
Figure 2-1. Zirconium oxide cell principle of operation.
Since the voltage of the cell is temperature dependent, the cell is main-
tained at a constant temperature. The oxygen content is then determined
from the Nernst equation:
E = In
RT
4F
O
1
O
2
where R and F are constants, T is absolute temperature, and O
1
and O
2
are
the oxygen partial pressures on either side of the cell.

 Loading...
Loading...











