Solvent miscibility D-3
Solvent miscibility
Before changing from one solvent to another, see the table below to determine
the miscibility of the solvents being used. The following considerations apply
when changing solvents:
• Changes involving two miscible solvents are made directly. Changes
involving two solvents which are not totally miscible (for example, from
hexane to water) require an intermediate solvent (such as methanol or
THF).
• Solvent miscibility is affected by temperature. If operating at an
elevated temperature, consider the effect of the higher temperature on
solvent solubility.
• Buffers dissolved in water can precipitate salts when mixed with organic
solvents. When switching from a strong buffer to an organic solvent,
flush the buffer out of the system with distilled water before adding the
organic solvent. Also, when using a strong buffer, flush all pathways
with distilled water before shutting the system down and leave distilled
water in the system (flush with 10% methanol in water for shutdowns
scheduled to be more than one day).
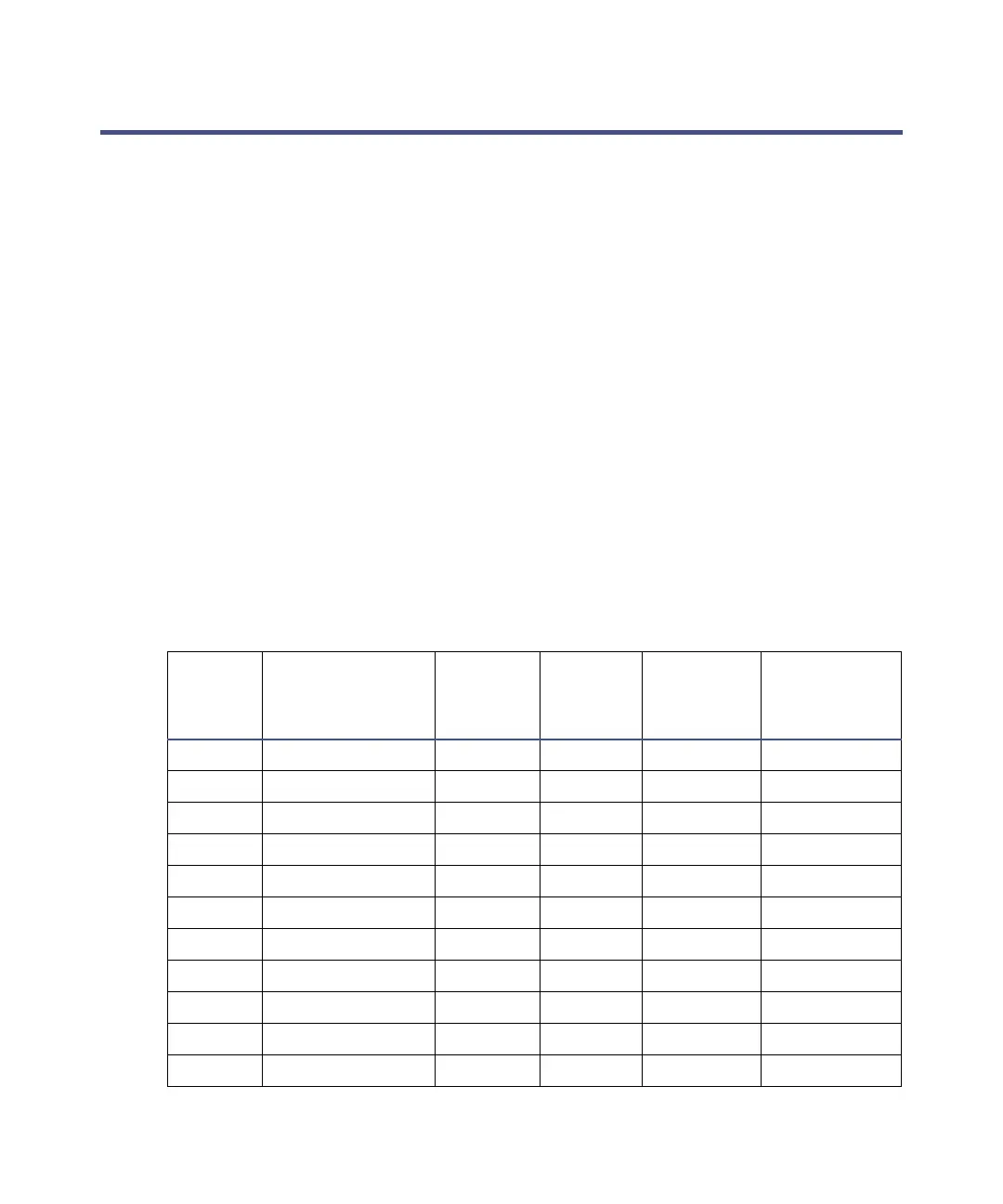
Physical properties of solvents
a
Polarity
index
Solvent
Viscosity
CP, 20 °C
Boiling
Point °C
(1 atm)
Miscibility
Number
(M)
Wavelength
Cutoffs (nm)
0.3 N-decane 0.92 174.1 29 —
0.4 Isooctane 0.50 99.2 29 210
0.0 N-hexane 0.313 68.7 29 —
0.0 Cyclohexane 0.98 80.7 28 210
C.7 Butyl ether 0.70 142.2 26 —
C.8 Triethylamine 0.38 89.5 26 —
2.2 Isopropyl ether 0.33 68.3 — 220
2.3 Toluene 0.59 10C.6 23 285
2.4 P-xylene 0.70 138.0 24 290
3.0 Benzene 0.65 80.1 21 280
3.3 Benzyl ether 5.33 288.3 — —
 Loading...
Loading...









