38
Publicaon 5423-020-REV 1.0 • www.btxonline.com
Pulse Length
The pulse length is the duraon of me the sample is exposed to
the pulse. This is measured as me in ranges from microseconds
to milliseconds. Adjusng this parameter is dependent on the
pulse waveform. The pulse length in a square wave system can be
inpued directly. The pulse length in an exponenal decay wave
system is called the “me constant” which is characterized by
the rate at which the pulsed energy (e) or voltage is decayed to
one-third the original set voltage. This me constant is modied
by adjusng the resistance and capacitance (RC) values in an
exponenal decay waveform. Time constant calculaon T = RC,
where T is me and R is resistance and C is capacitance.
The pulse length works indirectly with the eld strength to
increase pore formaon and therefore the uptake of target
molecules. Generally, during opmizaon of parameters an
increase in voltage should be followed by an incremental decrease
in pulse length. When decreasing the voltage, the reverse is true.
Pulse length is a key variable that works hand in hand with voltage
and needs to be considered when opmizing electrical parameters
to maximize the results for a given cell type.
Number of Pulses
Electroporaon is typically carried out as a single pulse for most
cell types. However, other cell lines may require mulple pulses
to achieve maximum transfecon eciencies. Usually lower
voltages are used when applying mulple pulses in order to
gradually permeate the cell membranes. This allows the transfer
of molecules while avoiding damage to delicate or whole ssue
samples. This method of mulple pulsing is crical for maximum
gene delivery without causing ssue damage to in vivo, in utero
and explant ssue environments. The use of mulple pulse will
require the opmizaon of key electrical parameters including
voltage and pulse length. Typically, for in vivo applicaons the use
of lower voltages between 10 and 100 volts with pulse lengths
ranging 30 to 50 ms provides ecient transfecon. The opmal
voltage, pulse length and number of pulses will vary depending on
the cell type and molecule (DNA or RNA) transfected.
Electroporaon Buer
The buers used for electroporaon can vary depending on the
cell type. Many applicaons use highly conducve buers such
as PBS (Phosphate Buered Saline <30 ohms) and HBSS (Hepes
Buer <30 ohms) or standard culture media which may contain
serum. Other recommended buers are hypoosmolar buers in
which cells absorbs water shortly before pulse. This swelling of
the cells results in lowering the opmal permeaon voltage while
ensuring the membrane is more easily permeable for many cells
but can be damaging to others. Prokaryoc cells such as bacteria
require the use of high resistance buers (>3000 ohms). For this
reason proper preparaon and washing of the cells is essenal
to remove excess salt ions to reduce the chance of arcing. Ionic
strength of an electroporaon buer has a direct aect on the
resistance of the sample which in turn will aect the pulse length
or me constant of the pulse. The volume of liquid in a cuvee
has a signicant eect on sample resistance for ionic soluons; the
resistance of the sample is inversely proporonal to the volume
of soluon and pH. As the volumes are increased resistance
decreases which increases the chance of arcing, Lowering the
volume will increase the resistance and decrease the arc potenal.
BTX oers BTXpress High Performance Electroporaon Soluon,
a low conductance buer that achieves higher transfecon
eciencies with minimal cell toxicity. The BTXpress buer is a
single buer developed to facilitate high eciency gene delivery
into mammalian cells.
DNA/RNA Concentraons
Electroporaon is typically thought of as a nucleic acid (DNA,
mRNA, siRNA and miRNA) transfer method into prokaryoc and
eukaryoc cells. Electroporaon is not limited to just nucleic acid
delivery, it can introduce proteins, anbodies, small molecules and
uorescent dyes.
The standard range of DNA used for transfecons is 5 – 20 µg/ml
for most cell types; however in some instances increasing the DNA
concentraon as high as 50 µg/ml improves transfecon eciency
without changing other parameters. Determining the opmal
DNA concentraon through a DNA traon can be benecial. The
size of a molecule will have an eect on the electrical parameters
used to transfect the cell. Smaller molecules (siRNA or miRNA)
may need higher voltage with microsecond pulse lengths and
larger molecules (DNA) may need lower voltages with longer pulse
lengths. Buers such as EDTA or Tris can drascally reduce the
transfecon eciency. Therefore, we recommend resuspending
DNA in dislled water. Finally, electroporang ligaon mixtures
into E. coli can cause arcing and reduced transformaons. Dilung
the ligaon mixture a minimum of 1:5 with diH
2
O, dialysis, or
ethanol precipitaon can signicantly improve transformaon
eciencies and reduce the potenal for arcing.
General Optimization Guide for Electroporation
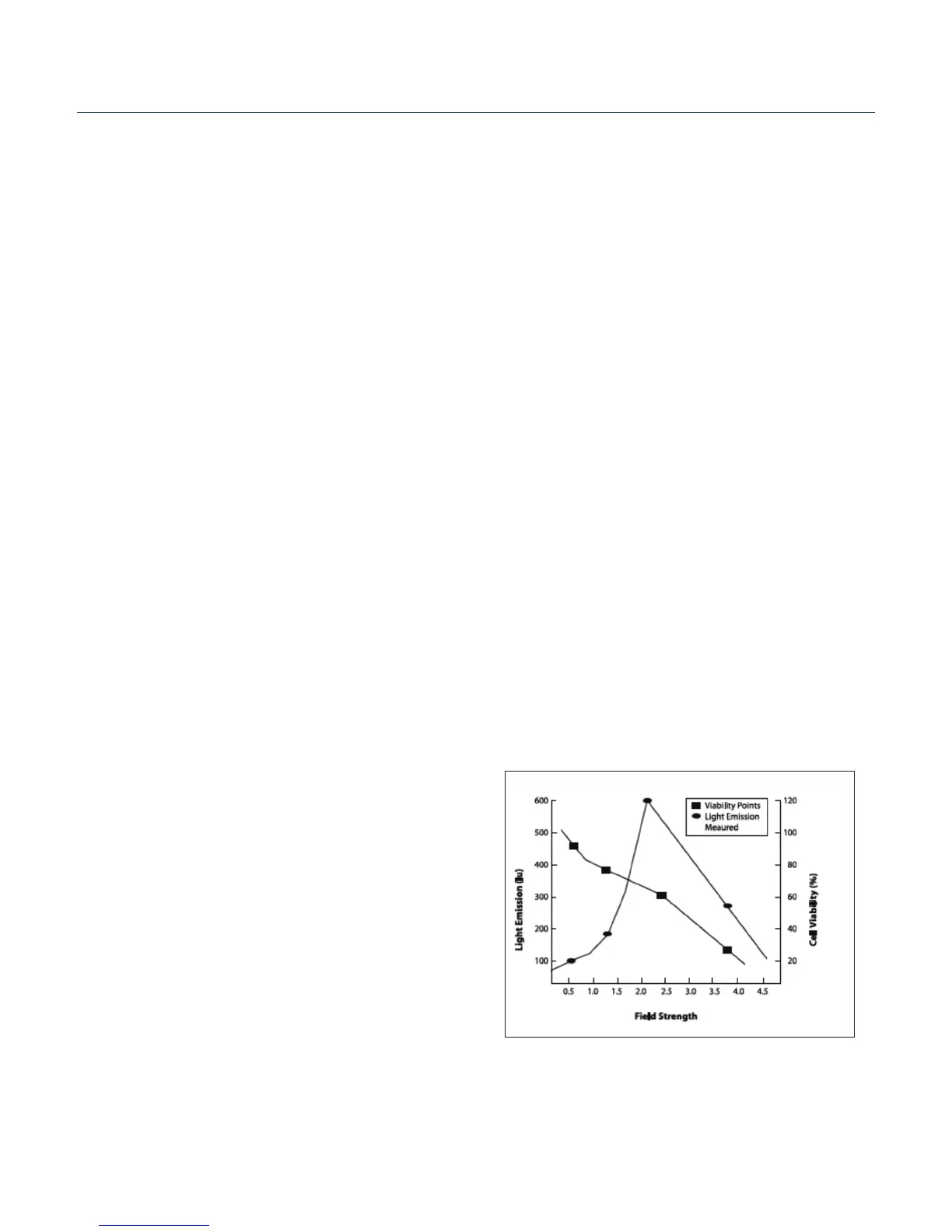
Protocol Opmizaon In Vitro
Choose the optimal eld strength based on the best conditions observed when
plotting viability versus expression at different eld strengths.
 Loading...
Loading...