143
Appendix A Specifications
Comply with EN 60601-1 (IEC 60601-1), Medical electrical equipment Part 1: General
requirements for basic safety and essential performance
EN 60601-2-37 (IEC 60601-2-37), Medical Electrical Equipment Part 2-37:
Particular Requirements for the Basic Safety and Essential Performance of
Ultrasonic Medical Diagnostic and Monitoring Equipment
EN 60601-1-2 (IEC 60601-1-2), Medical Electrical Equipment Part 1-2:
General requirements for basic safety and essential performance - Collateral
standard: Electromagnetic compatibility - Requirements and tests
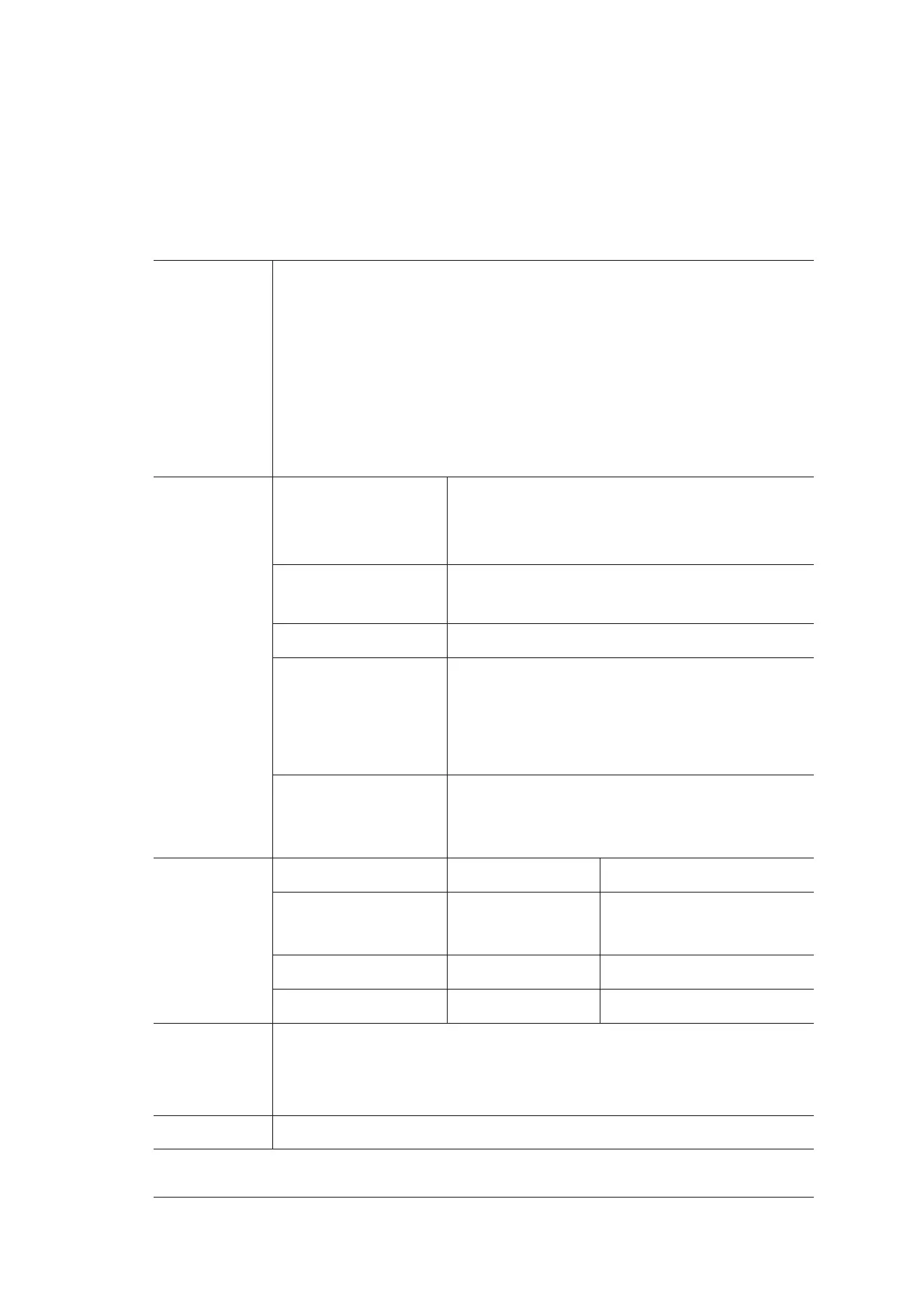
Classifications Type of protection
against electrical
shock
Class I ME equipment (with Adaptor);
Internally Powered ME equipment (when not
connected to supply mains)
Degree of protection
against electrical shock
Type-BF applied part
Installation Type Portable equipment
Degrees of protection
against harmful
liquid
●
System is IPX0
●
Probe (from the acoustic window to the junction
line) is IPX7
●
Foot switch is IPX8
According to the
degree of safety of
application
The equipment is not suitable for use in the presence
of a flammable anesthetic mixture with air, oxygen
or nitrous oxide.
Environmental
Requirement
Operations Storage and Transportation
Relative Humidity 30%-85%
(no condensation)
20%-90%
(no condensation)
Ambient Temperature 0°C to +40°C -20°C to +55°C
Atmospheric Pressure 700hPa-1060hPa 700hPa-1060hPa
Power supply AC/DC Adaptor:
Input: 100-240Va.c., 1.5-0.75A, 50-60Hz; Output: 19Vd.c., 4.74A
Main unit: 19Vd.c., 4.74A
Applied Parts Probe, ECG electrodes
 Loading...
Loading...