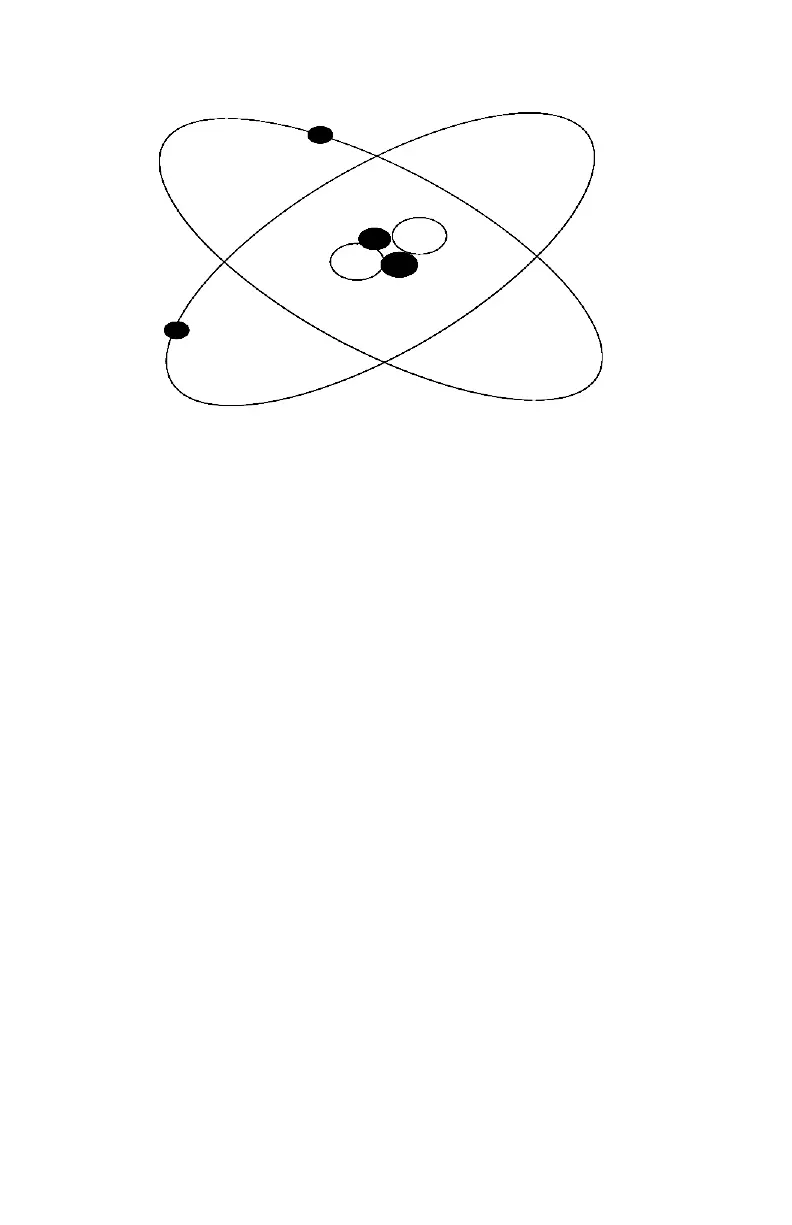
Figure 9. Diagram of an Atom
Protons carry a positive charge and are described as having a
mass of one. Neutrons have a neutral charge and have a mass of
one. Electrons carry a negative charge and essentially have no
mass.
Mass (Atomic Weight Scale)
Because protons and neutrons are clustered together in the
nucleus, the mass of an atom is concentrated in the nucleus. The
atom below has two protons and two neutrons; therefore, it is a
helium atom. The atomic weight of an atom is the sum of the
protons and neutrons.

 Loading...
Loading...