Overview | 1-3
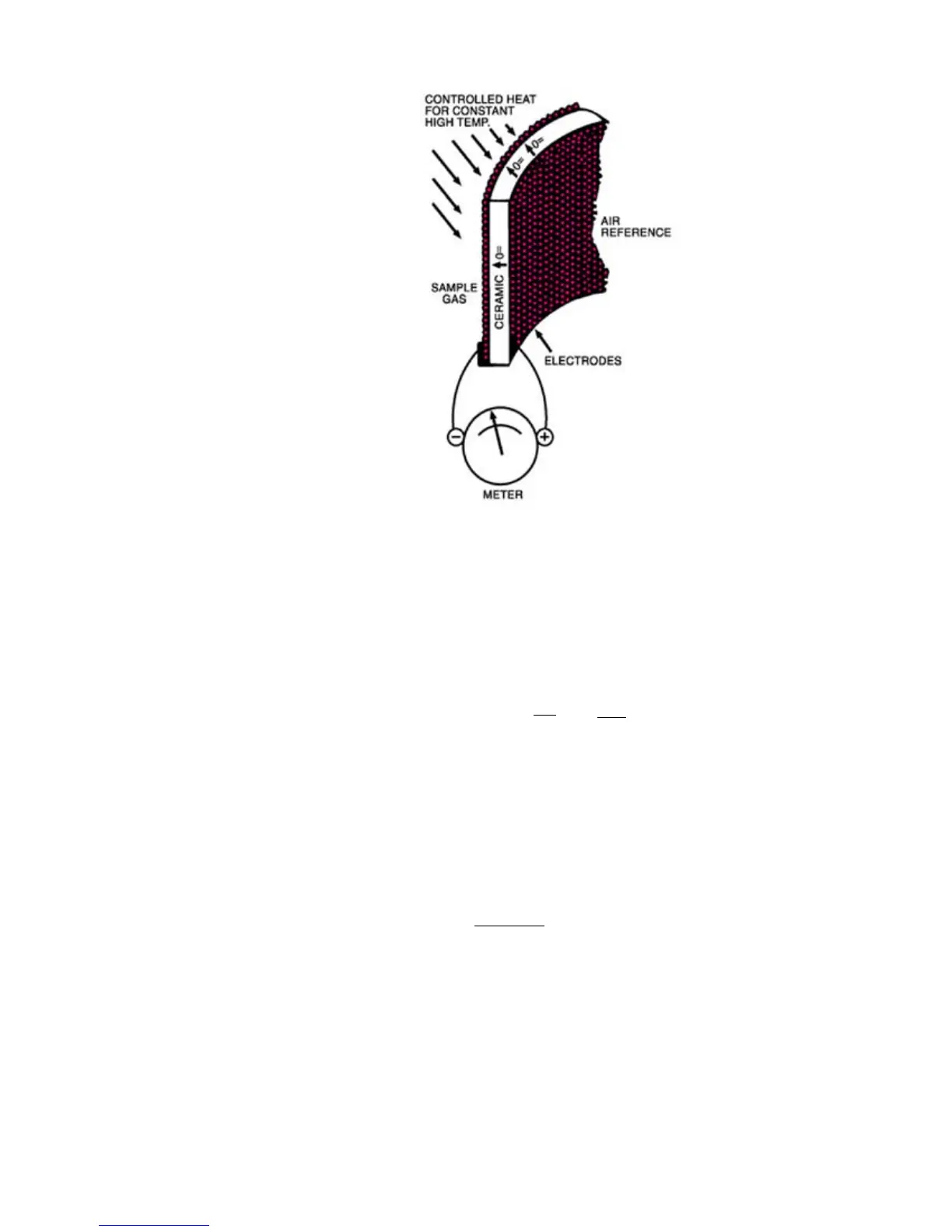
Figure 1-1. Zirconium oxide cell principle of operation.
Since the voltage of the cell is temperature dependent, the cell is main-
tained at a constant temperature. Some newer high temperature insitu
models use the heat from the process to heat the sensor, and the process
temperature is continuously measured and used in the software calcula-
tion. The oxygen content is then determined from the Nernst equation:
where R and F are constants, T is absolute temperature, and O
1
and O
2
are
the oxygen partial pressures on either side of the cell.
For measuring oxygen in non-combustibles gases, the calibration of an
analyzer is obtained from the formula:
AT = 48.0 at 695°C
E = A
*
T
*
Log
20.9%
O
2
Unk%
Where A is a constant, T is the cell temperature on an absolute scale (°C +
273) and O
2
Unk% is the unknown oxygen concentration of the gas to be
analyzed, and which is calculated by the analyzer.

 Loading...
Loading...











