Dexcom G5 Mobile System User Guide
317Technical Information
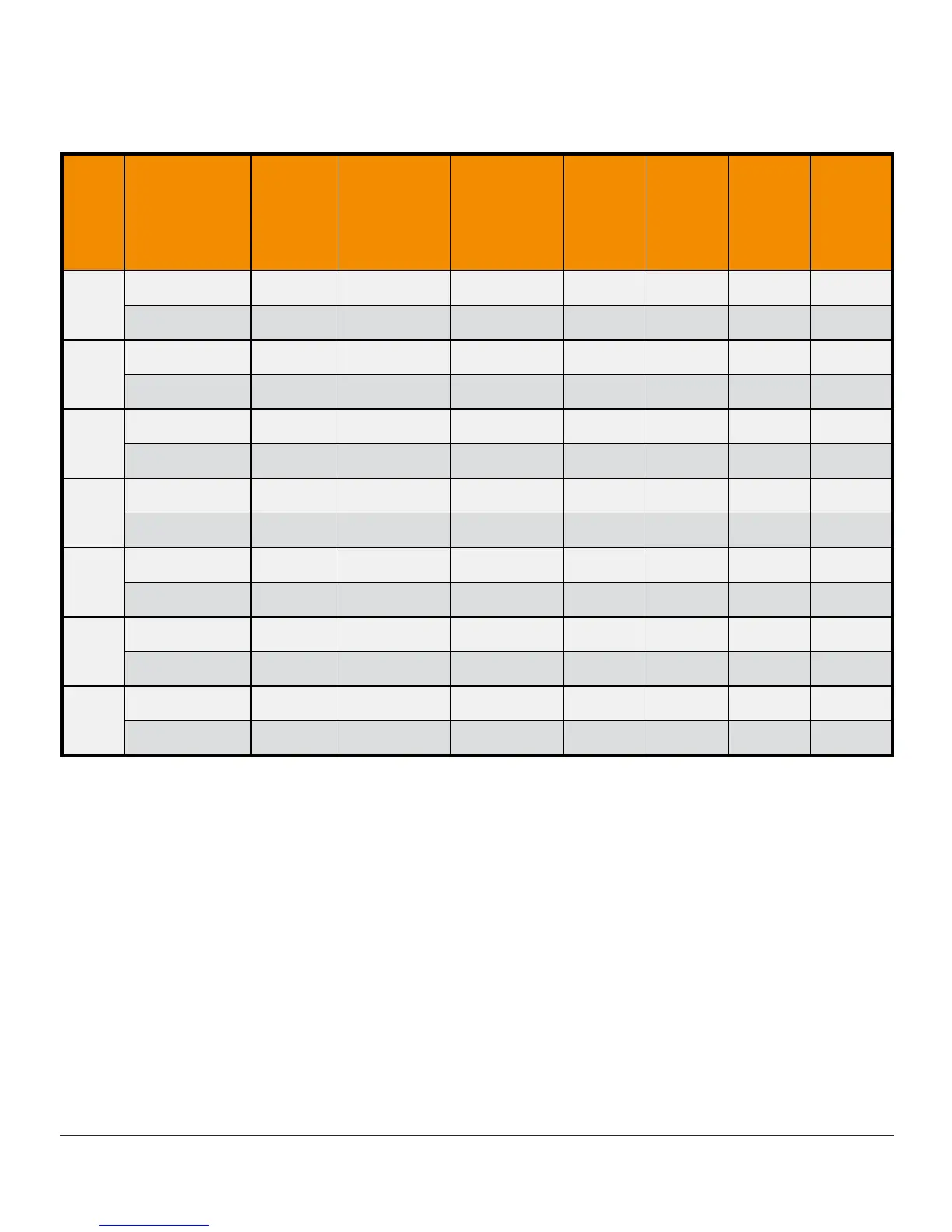
Table 8-C. Sensor Stability Relative to SMBG (Accuracy Over Time
1
) -
(Pediatric, Ages 2-17 Years)
Day
of
Wear
Study
2
Number
of
Paired
CGM-
SMBG
Mean
Absolute
Percent
Differences
Median
Absolute
Percent
Differences
Percent
Within
15/15%
SMBG
Percent
Within
20/20%
SMBG
Percent
Within
30/30%
SMBG
Percent
Greater
than
40/40%
SMBG
Day
1
Original 3216 18.8% 14.2% 53% 65% 81% 10%
Software 505 893 14.8% 10.7% 64% 79% 91% 5%
Day
2
Original 2148 16.2% 12.4% 60% 74% 87% 6%
Software 505 436 13.2% 10.4% 69% 81% 95% 3%
Day
3
Original 1977 15.2% 11.0% 63% 76% 89% 5%
Software 505 441 13.8% 11.3% 66% 77% 91% 2%
Day
4
Original 2830 14.0% 10.9% 66% 79% 91% 4%
Software 505 850 10.7% 8.5% 79% 91% 97% 1%
Day
5
Original 1768 15.4% 10.7% 67% 78% 90% 5%
Software 505 374 11.4% 8.7% 74% 86% 96% 1%
Day
6
Original 1704 14.3% 9.8% 68% 79% 90% 4%
Software 505 410 12.3% 9.2% 72% 80% 93% 2%
Day
7
Original 2675 12.4% 9.2% 72% 83% 94% 3%
Software 505 860 11.3% 8.6% 79% 90% 96% 2%
1
CGM readings are within 40 to 400 mg/dL, inclusive.
2
Both sets of study data are presented and are labeled as Original (SW10050) or Software 505
(SW10505).
Sensor Stability
Relative to YSI
Sensors can be worn for up to 7 days. Performance was estimated by calculating the percentage of
System readings within 15 mg/dL or 15%, 20 mg/dL or 20%, 30 mg/dL or 30% , 40 mg/dL or 40%
and greater than 40 mg/dL or 40% of the YSI values at the beginning (Day 1), middle (Day 4) and
end (Day 7) of the System lifecycle. The average and median of the absolute percent differences are
 Loading...
Loading...