GE HEALTHCARE
DIRECTION 5394227, 12 LOGIQ S8/LOGIQ E8 SERVICE MANUAL
1 - 14 Section 1-3 - Important conventions
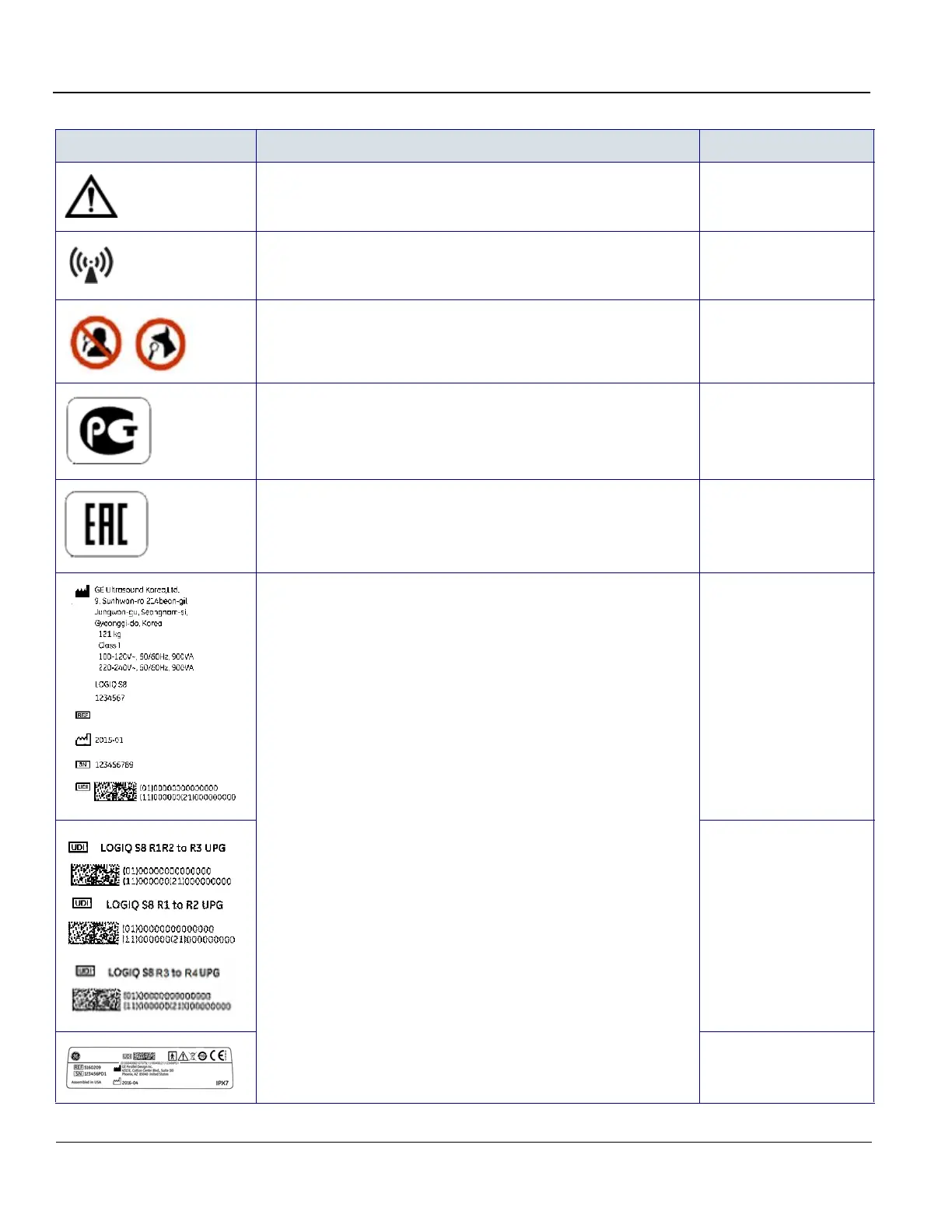
Caution Probe connector
Non-Ionizing Electromagnetic Radiation
Neck of Monitor arm, on
equipped with Wireless
LAN
This system is for animal use only. Do not mix up with human
diagnosis.
Rear of the LS8 Vet
system
GOST Symbol. Russia Regulatory Country Clearance. Under the rating plate
EAC Mark of Conformity. Image of the Common Mark of Products
Circulation in the market of the Customs Union member-states.
Under the rating plate
Every system has a unique marking for identification, the Unique Device
Identification (UDI) Label. The UDI label consists of a series of alpha-numeric
characters and barcode which uniquely identify the LOGIQ S8 system as a
medical device manufactured by General Electric. Scan or enter the UDI
information into the patient health record as required by country-specific laws.
Rating Plate
Upgraded LOGIQ S8
systems, UDI label is on
the left-rear caster.
Probe UDI label
Table 1-7 Label Icons(Continued)
Label/Icon Purpose/Meaning Location
 Loading...
Loading...