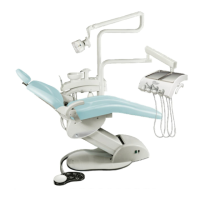
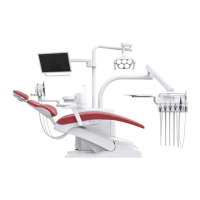
Care instructions KaVo uniQa
2 Reprocessing steps in accordance with ISO 17664 | 2.1 Notes
7 / 56
2 Reprocessing steps in accordance with ISO 17664
2.1 Notes
The reprocessing consists of the following basic steps:
▪ Cleaning and disinfection ( manually or automatically)
▪ Sterilisation
NOTE
Comply with national hygiene requirements, e.g. RKI guidelines (RKI = Ger-
man Robert Koch Institute).
CAUTION
Risk of injury during cleaning of the treatment centre.
Lack of instructions to the cleaning staff and lack of preparation of the treat-
ment centre can lead to the cleaning personnel sustaining injuries.
4 Only trained professionals and instructed cleaning personnel may be present
in the treatment rooms.
4 Position the chair for cleaning and turn the device off.
CAUTION
Improper sterilisation may lead to health hazards and material dam-
age.
Infection hazard to users and patients. Damage to the sterilised items.
4 No hot air sterilisation, no chemical cold sterilisation, do not sterilise with
ethylene oxide.
CAUTION
Health hazard and property damage due to non-compliance with servi-
cing schedule.
Infection hazard to users and patients. Product damage.
4 Comply with servicing schedule.
CAUTION
Working with cleaning, germ reduction and disinfection agents neces-
sitates a certain degree of diligence.
Risk of injury from handling cleaning, germ reduction and disinfection agents.
4 It is essential to follow the notes shown on the bottle label and in the mater-
ial safety data sheet.
4 Use personal protective equipment when you use these agents.
CAUTION
Product damage due to improper disinfection.
Malfunctions.
4 Use the disinfectant according to the specifications of the manufacturer.
4 No spray disinfection, perform wipe disinfection only.
4 Do not immerse product or parts of the product in liquids.
4 Mop up any spilled cleanser or disinfectant immediately.
 Loading...
Loading...