56
MAGNAMED TECNOLOGIA MÉDICA S/A
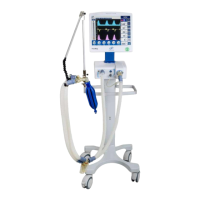
11 Technical specifications
Equipment Classification
11.1.1 Risks
✓ As MERCOSUL/GMC/RES. Nº 40/00: Class III
11.1.2 Electrical isolation
✓ As ABNT NBR IEC 60601-1:1994
✓ Class II
✓ Applied part type BF
✓ Energized equipment internally
✓ Part applied to proof defibrillation
11.1.3 Mode of operation
✓ As ABNT NBR IEC 60601-1:1994
✓ Equipment for use in continuous operation
11.1.4 Protection against liquid penetration
✓ As ABNT NBR IEC 60601-1:1994
✓ Ingress Protection IPX1
Applicable standards
• IEC 60601-1 (1988) + Amd. 1 (1991) + Amd. 2 (1995), IEC 60601-1-1 (2000) (EN 60601-1:2006 +
A1:2013) - Medical electrical equipment - Part 1: General requirements for basic safety and essential
performance
• IEC 60601-2-12: 2001 (EN 60601-2-12:2006) - Medical electrical equipment - Part 2-12: Particular
requirements for the safety of lung ventilators - Critical care ventilators
• ISO 5359:2008/Amd 1:2011 (EN ISO 5359:2008+A1:2011) - Low-pressure hose assemblies for use
with medical gases
• IEC 60601-1-2 Ed. 3.0 (2007) (EN 60601-1-2:2007) - Medical electrical equipment - Part 1-2: General
requirements for basic safety and essential performance - Collateral standard: Electromagnetic
compatibility - Requirements and tests
• IEC 62304:2006 +AMD1:2015 (EN 62304:2006/2008) - Medical device software - Software life-cycle
processes
• IEC 60601-1-8 Ed. 2.0 (2006)/A1:2012 (EN 60601-1-8:2007/A11:2017) - Medical electrical equipment
- Part 1-8: General requirements for basic safety and essential performance - Collateral Standard:
General requirements, tests and guidance for alarm systems in medical electrical equipment and
medical electrical systems
 Loading...
Loading...