66
GENERAL INFORMATION ON USAGE AND MAINTENANCE
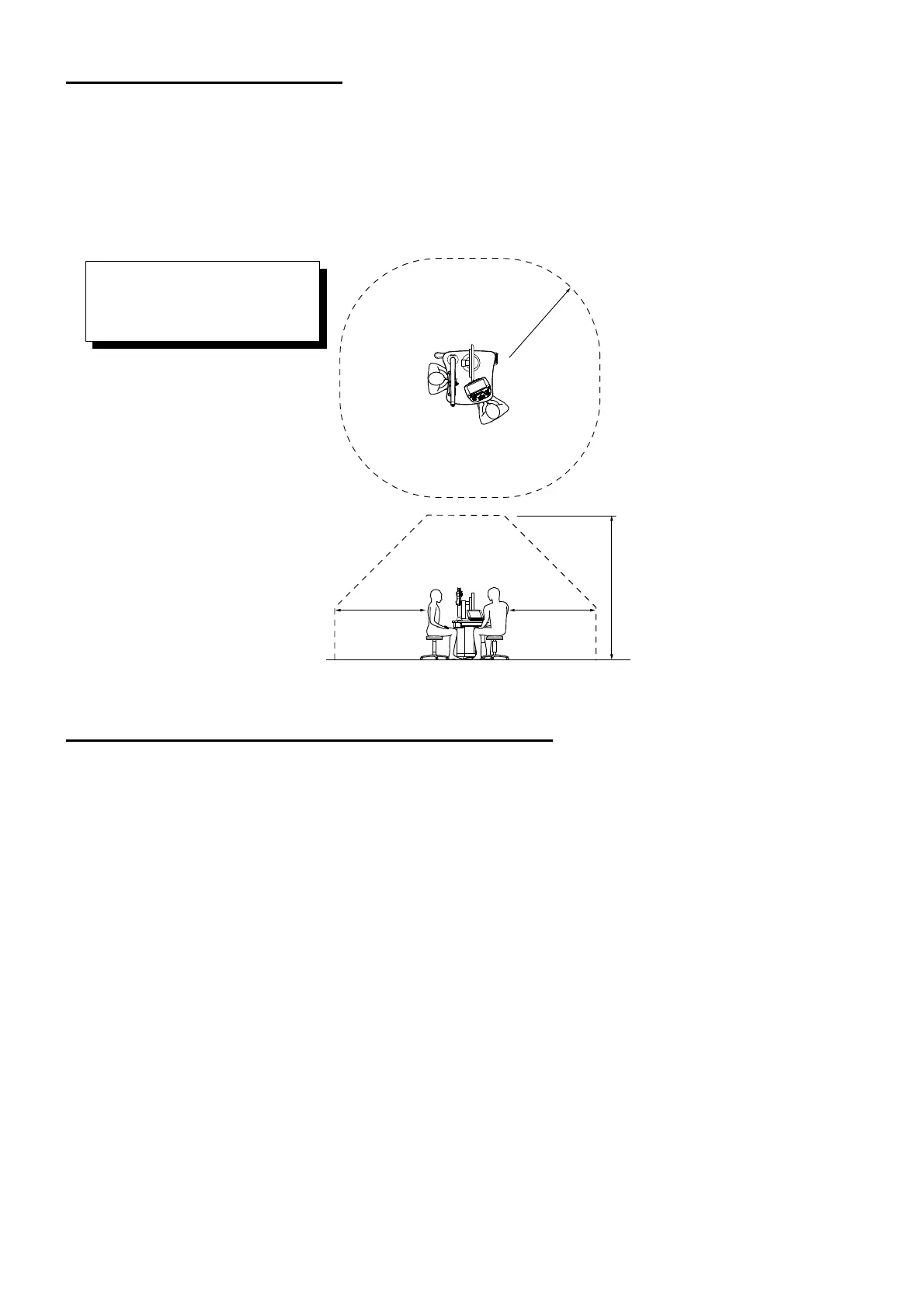
PATIENT’S ENVIRONMENT
When the patient or inspector may touch the devices (including the connecting devices) or when the
patient or inspector may touch the person in contact with the devices (including the connecting
devices), the patient's environment is shown below.
In the patient's environment, use a device conforming to IEC60601-1. If you are compelled to use any
device not conforming to IEC60601-1, use an insulation transformer or the common protective earth
system.
REQUIREMENTS FOR THE EXTERNAL DEVICE
The external device connected to the analog and digital interfaces must comply with the respective IEC
or ISO standards (e.g. IEC 60950 for data processing equipment and IEC 60601-1 for medical equip-
ment).
Anybody connecting additional equipment to medical electrical equipment configures a medical system
and is therefore responsible that the system complies with the requirements for medical electrical sys-
tems. Attention is drawn to the fact that local laws take priority over the above mentioned requirements.
If in doubt, contact your dealer or TOPCON(see the back cover).
Radius 1.5m
1.5m1.5m
2.5m
Device applicable to the use in
• USB memory
Do not use a power strip in the
patient's environment. Connect
the power supply of the device to
the commercial power supply.
patient’s environment
 Loading...
Loading...