Foreword
13
Osmo1® Single-Sample Micro-Osmometer User Guide
Instrumentation
Advanced Instruments Osmometers measure the freezing point of an aqueous solution to determine solute concentration.
Advanced Instruments Osmometers utilize high precision thermistors to sense the sample temperature, to control the
degree of super cooling and freeze induction, and to measure the freezing point of the sample. The thermistor can
determine dierences of ±1 mOsm/kg H
2
O.
Freezing-point thermodynamics
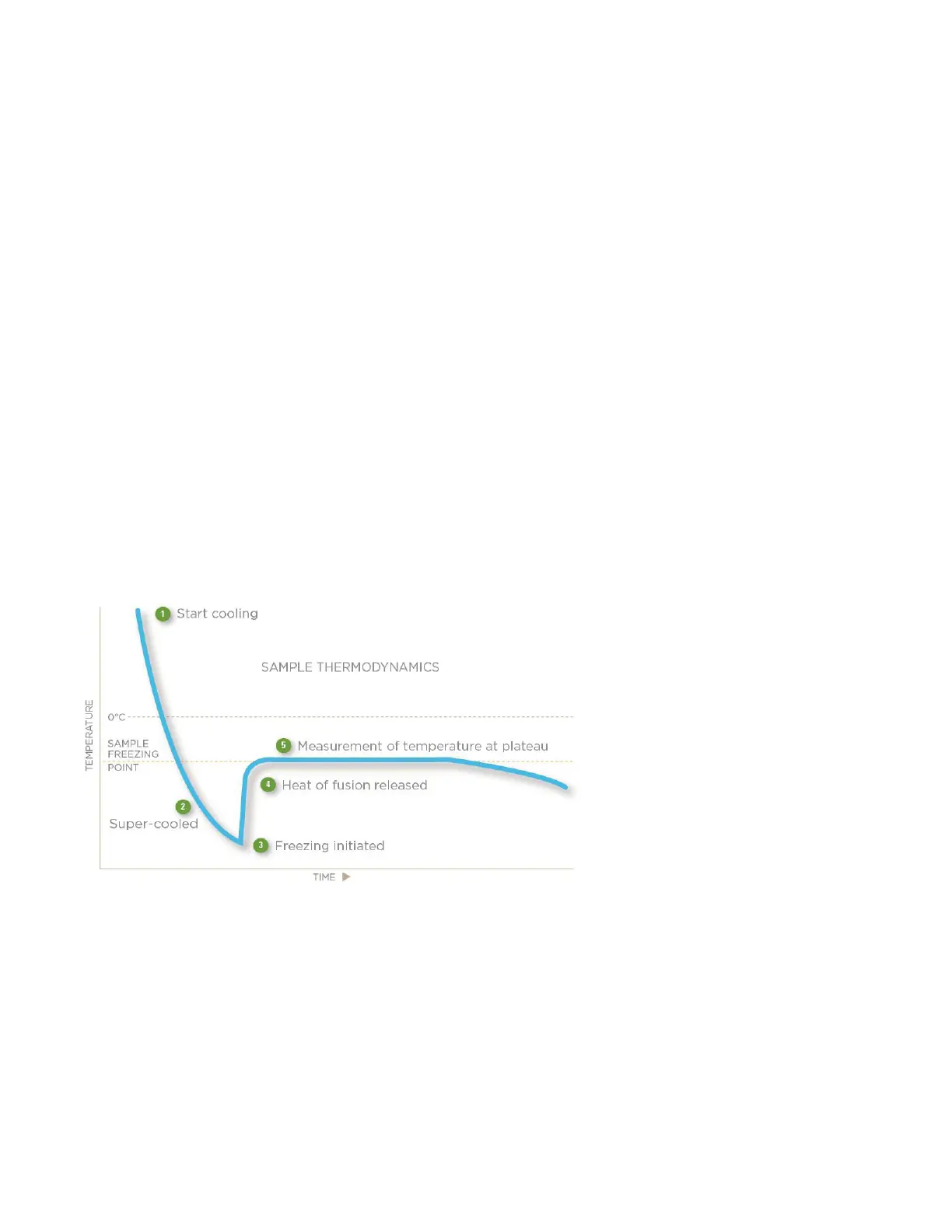
The quickest and most precise way to measure the freezing point of a solution is to supercool it several degrees below its
freezing point and inducing crystallization of the solution via mechanical agitation. The sudden liberation of energy (heat
of fusion) causes the sample temperature to rise toward a plateau temperature, where a liquid/solid equilibrium occurs.
The equilibrium temperature is the freezing point of the solution.
The duration of the liquid/solid equilibrium phase is a function of the speed at which the heat of fusion is liberated versus
the speed at which it is dissipated to the surrounding environment. This ratio can be slowed to prolong the equilibrium
time, giving a distinct plateau measurable to 0.001 °C.
Sensitive thermistor probes monitor the sample temperature and control the thermoelectric cooling element.
Microprocessor control and automated operation minimize imprecision introduced by operator technique.
The standard freezing curve shown below (Figure 1) illustrates the temperature of a sample as it progresses through the
freezing cycle and shows the action of the osmometer at each stage of the cycle.
Figure 1: Standard freezing curve

 Loading...
Loading...