TABLE OF CONTENTS
i
Vectra Genisys® Therapy System
FOREWORD ............................................... 1
PRODUCT DESCRIPTION.....................................1
SAFETY PRECAUTIONS.................................. 2-12
PRECAUTIONARY DEFINITIONS .............................2
Caution ...........................................................2
Warning ..........................................................2
Danger............................................................2
Dangerous Voltage ...............................................2
Corrosive .........................................................2
Spontaneous Combustion .......................................2
Biohazardous Materials ..........................................2
Non-Ionizing Electromagnetic Radiation........................2
CAUTIONS ...................................................3
WARNINGS...................................................4
DANGERS ....................................................6
ELECTROTHERAPY INDICATIONS, CONTRAINDICATIONS,
AND ADVERSE EFFECTS .....................................7
Indications for VMS, VMS Burst, Russian, TENS,
High Voltage Pulsed Current (HVPC), Interferential,
and Premodulated Waveforms...................................7
Additional Indications for Microcurrent, Interferential,
Premodulated, VMS™, VMS™ Burst, and TENS Waveforms .......7
Indications for DC (Direct Current) Mode ........................7
Contraindications ................................................7
Additional Precautions ...........................................8
Adverse Eects ...................................................8
sEMG INDICATIONS ..........................................9
sEMG + STIM INDICATIONS, CONTRAINDICATIONS
AND ADVERSE EFFECTS ....................................10
Indications- sEMG + Stim using VMS™, Symmetrical Biphasic
(TENS), Asymmetrical Biphasic (TENS), or Russian waveforms .10
Contraindications ...............................................10
Additional Precautions ..........................................11
Adverse Eects ..................................................11
ULTRASOUND INDICATIONS AND CONTRAINDICATIONS ..12
Indications for Ultrasound ......................................12
Contraindications ...............................................12
Additional Precautions ..........................................12
NOMENCLATURE ......................................13-17
VECTRA GENISYS ELECTROTHERAPY AND
COMBINATION THERAPY SYSTEMS.........................13
Two Channel Electrotherapy System............................13
Two Channel Combination System..............................13
Front Access Panel...............................................14
Rear Access Panel................................................14
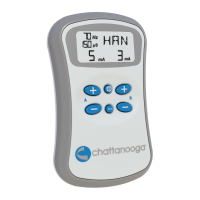
USER INTERFACE............................................15
SYMBOL DEFINITIONS......................................16
System Hardware Symbols ......................................16
System Software Symbols.......................................16
Optional Module and Accessory Symbols ......................16
Operator Remote................................................16
Battery Module ..................................................16
Channel 3/4 Electrothrapy Module .............................16
GENERAL TERMINOLOGY...................................17
Back Button......................................................17
Previous Page Button ...........................................17
UP and DOWN Arrows...........................................17
Electrotherapy...................................................17
 Loading...
Loading...