GE
D
IRECTION 5535208-100, REV. 2 LOGIQ E9 SERVICE MANUAL
Chapter 8 Replacement procedures 8 - 51
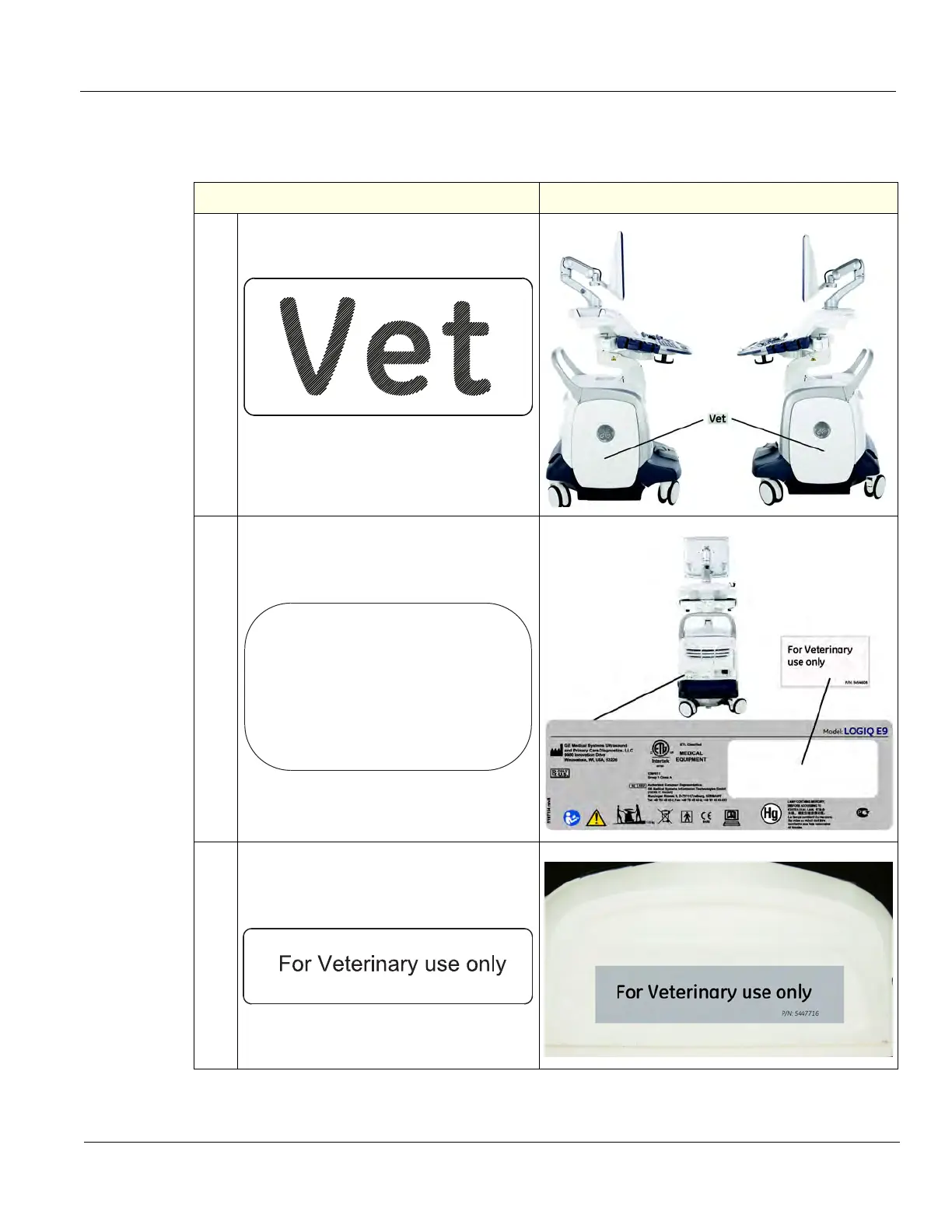
8-5-8-5 Label Placement for the LOGIQ E9 used in a Veterinary Environment
Table 8-36 LOGIQ E9 used in a Veterinary Environment Label Placement
Steps
Corresponding Graphic
1. If the Side Cover Assemblies are replaced,
reinstall the “VET“ Label as shown.
1.
If the Rear Cover Assembly is replaced,
reinstall the “For Veterinary use only“ Label
for the Rear Cover Assembly as shown.
NOTE: The Back Cover Label with ETL
Label is not a Spare Part.
2.
If a Probe is replaced, reinstall the “For
Veterinary use only“ Label for Probes as
shown.
For Veterinary
use only
5454608
5447716
 Loading...
Loading...