Page 6 199253
(6) -
EN
-
Accella™ Therapy Mattress Instructions for Use
For the mattress P006789A* or P006792A* (combined with the Accella™ bed)
and P006793A* (combined with the Progressa™ bed), refer to the bed’s manual
for the recommendations on connections.
In compliance with standards relating to electromagnetic interference for medical
devices, this product does not interfere with other medical devices nor is it
susceptible to interference when combined with other medical devices that also
comply with the electromagnetic standards in place.
However, some devices, particularly older ones that do not comply with the
electromagnetic compatibility standards, may undergo interference or may
themselves interfere with the functionality of this device.
Using accessories or cables other than those specified, with the exception of cables
sold by the device manufacturer, as replacement parts for internal components, may
result in an increase of emissions or a reduction of the device's immunity.
The users of such devices are responsible for ensuring that any malfunctions will not
endanger the patient or any other person.
When intravascular or intracardiac connections are in use, the electric potentials of all
the unprotected metal parts of the appliance and the bed need to be equalized.
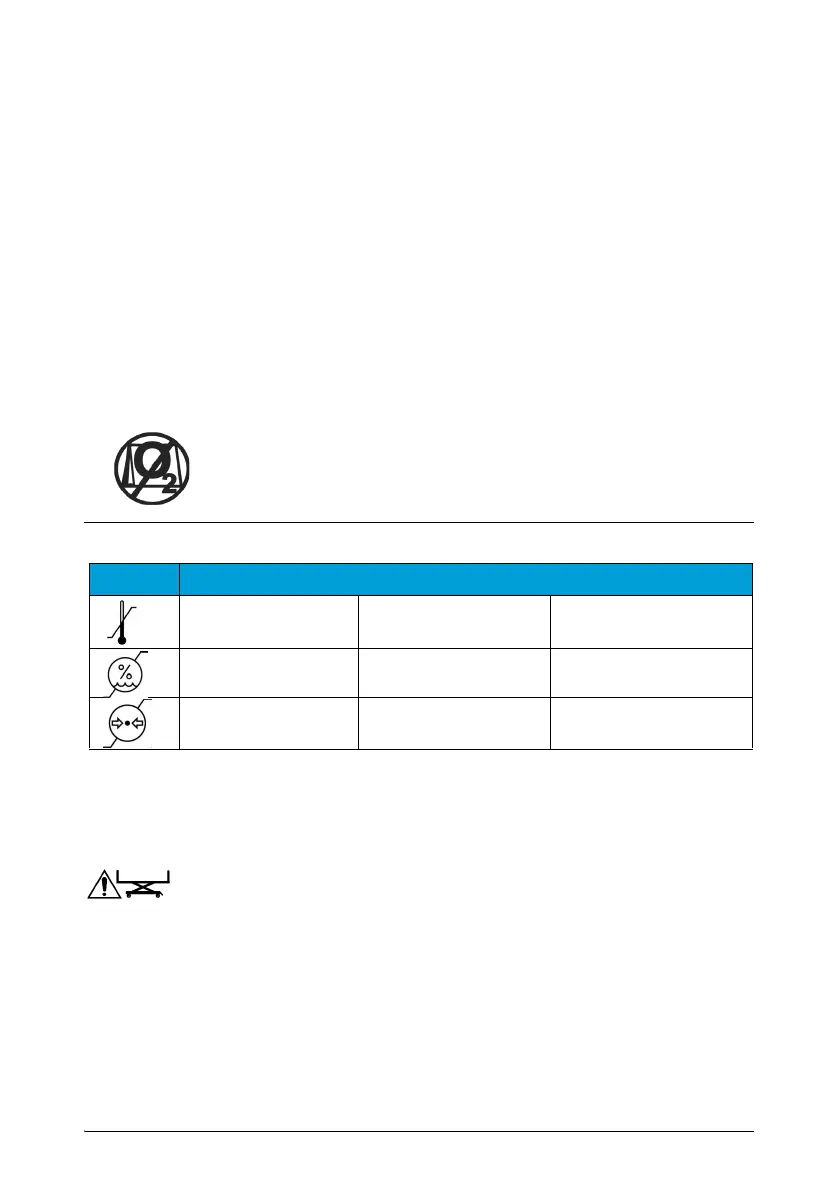
This label indicates that
oxygen tents must never be used
and that
only the use of nasal tubes and oxygen masks is authorized. For reasons
of safety, masks and tubes should always be kept at a higher level than
the mattress support platform.
Complying with Conditions for Transport, Storage and Use
The device must be stored in its original packaging:
• protected against light and damp;
• At least 10 cm above floor level to prevent fluid ingress;
• protected against dust;
• outside passageways.
Never stack more than 5 mattresses.
Symbol Features Use
Transport/storage
a
a. Applicable only if the device is stored in its original packaging.
The device is designed for indoor use only. When used at 40°C,
the temperature of the applied part can reach 43°C. The electrical medical device
must be used at an altitude of 3000 m or lower.
Temperature +10°C to +40°C -30°C to +50°C
Hygrometry 30% - 85% 20% - 85%
Atmospheric
pressure
700 mbar - 1060 mbar 700 mbar - 1060 mbar

 Loading...
Loading...