199253
(6) -
EN
-
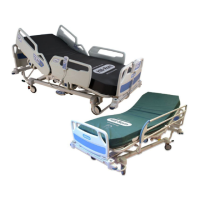
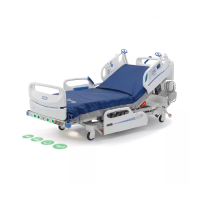
Accella™ Therapy Mattress Instructions for Use Page 3
Destination and
Specifications
Safety and Usage Tips
Intended Use
This device
benefits are
phase I to phase IV pressure ulcers prevention and
treatment
help.
Indications
It is suitable for low to very high-risk patients, within the recommended patient weight
limits of 30 to 160 kg on the standalone mattress and between 40 and 160 kg on the
version combined with the Accella™ bed or
Progressa™ bed
, in order to achieve
valuated clinical performance in all the usual positions of the adjustable head section.
It can be used as a mattress in the following environments, as defined in the standard
IEC 60601-2-52:
• application environment 1 (acute care);
• application environment 2 (short-term care in hospitals or other medical
establishments);
• application environment 3 (long-term care in medical establishments);
• application environment 5 (outpatients or ambulatory care).
The device
is not designed to come into direct contact with damaged skin
and
must to be used with a sheet between the patient’s skin and the surface of the
mattress.
In accordance with the NPUAP/EPUAP directives
1
, Hill-Rom recommends that the
condition of each patient be regularly checked. For patients with special needs, Hill-Rom
recommends the use of the most suitable I-mmersion™ Therapy system. Caregivers are
responsible for taking this decision, in accordance with modern care practices.
Contraindications
This device must not be used with patients:
• Suffering from an unstable fracture of the spine; for any other unstable
fractures, a medical examination is necessary to determine whether the use
of the device is appropriate.
• with atypical anatomy;
• suffering from cervical or trans-bone traction.
Intended Users
The Accella™ Therapy mattresses are designed to be used by Qualified Staff for
patient care from several care application environments.
1. NPUAP / EPUAP - Prevention and treatment of Pressure Ulcers - Quick Reference Guide, 2019

 Loading...
Loading...