Plate Specification Editor
Source plates require very exacting specifications to accurately transfer nanoliter volumes; therefore, they are defined specifically
for the Echo instruments and tested as Labcyte Echo® qualified microplates. For this reason, new source plates cannot be defined
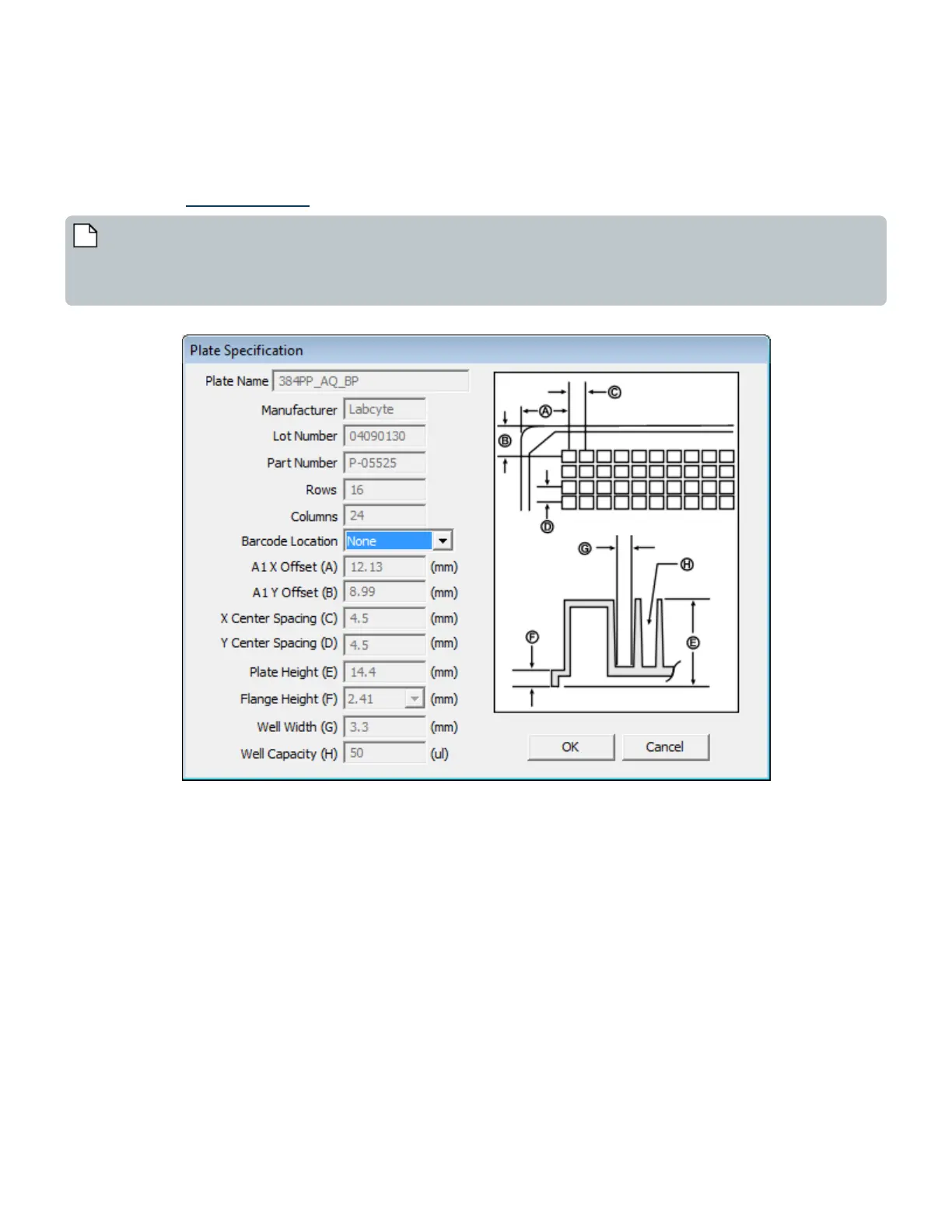
by the user. For existing source plates, only the barcode location can be edited (see figure below). Contact Labcyte to add more
source plates. See Contact Information for more information.
Note: In Echo application software, such as Echo Dose-Response and Echo Cherry Pick, (version 1.5 or later), existing
source plates can be copied and renamed, to allow for more specific identification for application workflows, and then
imported into Echo Liquid Handler software. However, other plate parameters cannot be changed--except for barcode
location.
Figure 67: Plate Specification for Source Plate
Source plate specifications cannot be removed from the Echo Liquid Handler software or the Plate Name List. All source plate
specifications can be used as destination plate specifications; however, no user-defined destination plate specification can be
used as a source plate specification.
111 PN | 001-11665
USER GUIDE | Echo® 525 Liquid Handler User Guide Echo Liquid Handler Software
 Loading...
Loading...