4040+e_22.docx / 06.10.17
ROBERT RIELE GmbH & Co KG Page 2 GENERAL NOTES
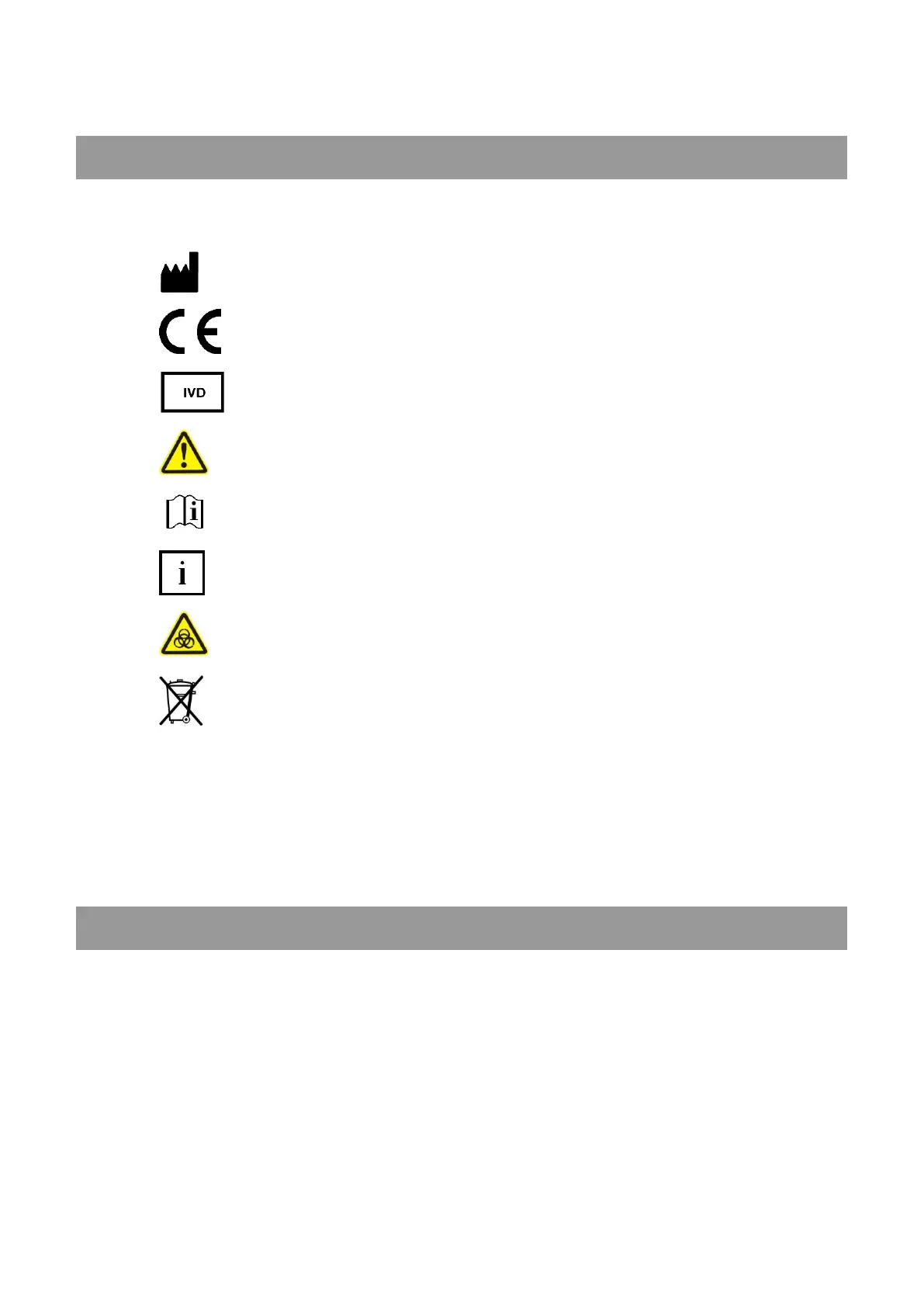
SYMBOLS
The packaging material, the type plate on the instrument and the manual may contain the following symbols or
abbreviations:
This product fulfills the requirements of Directive 98/79/EC on
in vitro diagnostic medical devices.
In vitro diagnostic medical device
Caution (refer to accompanying documents)!
Please refer to safety-related notes in the manual accompanying this device.
Please consult instructions for use
Symbol for the marking of important information for appropriate handling of the device
Biohazard
Samples containing material of human origin must be treated as potentially infectious.
The relevant laboratory guidelines on safe use must be observed.
Symbol for the marking of electrical and electronics devices according to § 7 ElektroG
No special protection against penetrating moisture (IP = International Protection)
INSTRUMENT APPROVALS
The Photometer 4040
+
meets the requirements of Directive 98/79/EC on in vitro diagnostic medical devices
(IVDD). Furthermore, the Photometer 4040
+
is manufactured according to the special safety requirements for IVD
medical devices stated in EN 61010.
 Loading...
Loading...