36 Principles of the 2996 PDA Detector Optics
4
Beer’s Law
The relationship between the quantity of light of a particular wavelength arriving at the
photodiode and the concentration of the sample passing through the flow cell is described
by the Beer-Lambert Law (commonly called Beer’s Law). Beer’s Law is expressed as:
A = εlc
where:
A = absorbance
ε = molar absorptivity
l = path length (1.0 cm in the 2996 Detector normal flow cell)
c = molar concentration
Beer’s Law applies only to well-equilibrated dilute solutions. It assumes that the refractive
index of the sample remains constant, that the light is monochromatic, and that no stray
light reaches the detector element. As concentration increases, the chemical and
instrumental requirements of Beer’s law may be violated, resulting in a deviation from
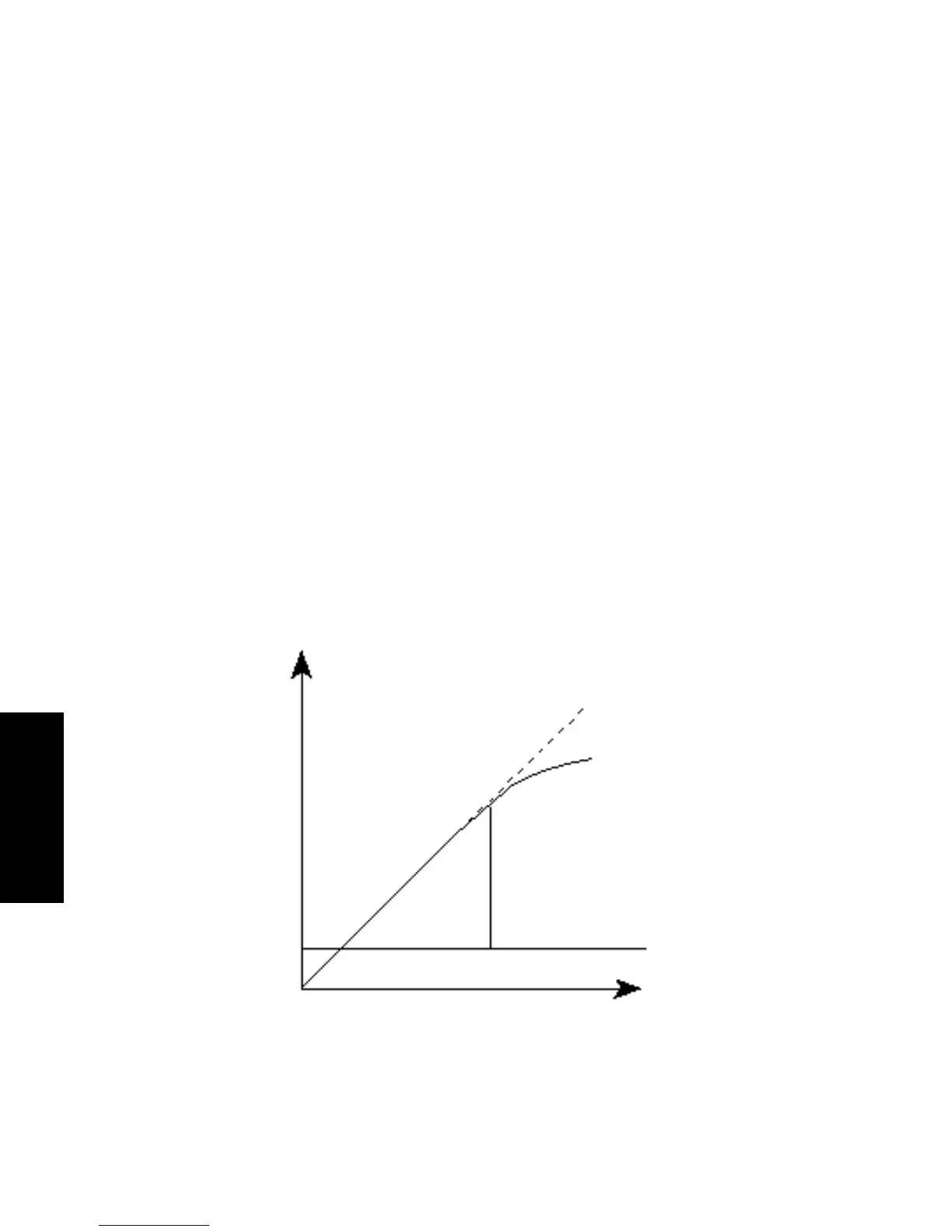
(absorbance versus concentration) linearity (Figure 4-4). The absorbance of mobile phase
can reduce the linear range by the amounts shown in Appendix C, Mobile Phase
Absorbance.
Figure 4-4 Absorbance as a Function of Concentration
Ideal
Actual
Absorbance
Working Range
Background Absorbance
Concentration
 Loading...
Loading...