<9. Calibration>
9-2
IM 11M12A01-04E 11th Edition : Jul. 19, 2017-00
0.1
0.5 1 5 10 21.0
50 100
120
100
80
60
40
20
0
-20
-40
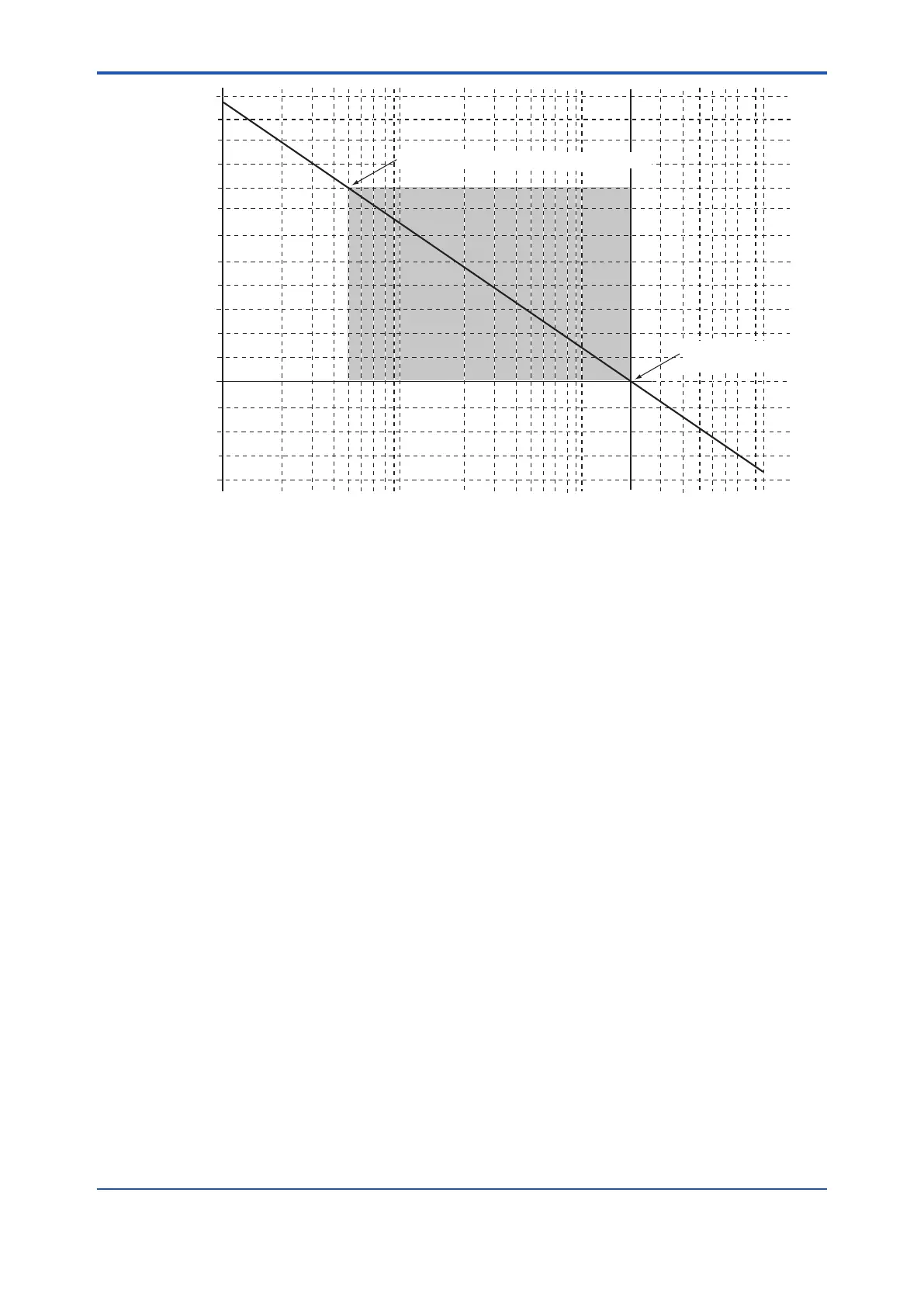
0.51 vol%O2,81.92mV(Zero origin of calibration)
21.0 vol%O2, 0mV
(Span origin of calibration)
Oxygen concentration (vol % O
2
F9.1E.ai
Figure 9.1 Oxygen Concentration in a Measurement Gas vs. Cell Voltage (21 vol%O
2
Equivalent)
The measurement principles of a zirconia oxygen analyzer have been described above.
However, the relationship between oxygen concentration and the electromotive force of a cell
is only theoretical. Usually, in practice, a sensor shows a slight deviation from the theoretical
value. This is the reason why calibration is necessary. To meet this requirement, an analyzer
calibration is conducted so that a calibration curve is obtained, which corrects the deviation from
the theoretical cell electromotive force.
9.1.2 Measurement Principle of Zirconia Humidity Analyzer
A solid electrolyte such as zirconia allows the conduction of oxygen ions at high temperatures.
Therefore, when a zirconia-plated element with platinum electrodes on both sides is heated up
in contact with gases having different partial-oxygen pressures on each side, oxygen ions ow
from a high partial-oxygen pressure to a low partial-oxygen pressure, causing a voltage. When
a sample gas introduced into the zirconia-plated element with the measurement electrode, and
air (21.0 vol % O2) is owed through the reference electrode, an electromotive force (mV) is
produced between the two electrodes, governed by Nernst’s equation as follows:
E = - RT/nF log e y/a ………………………… Equation (1)
where, R = Gas constant
T = Absolute temperature
n: 4
F = Faraday’s constant
y = O2 vol % on the zirconia element measurement electrode
a = O2 vol % to 21.0 vol % O2 on the zirconia element reference electrode
The humidity analyzer uses a sample gas composed of water vapor and air.
(A) For the vol % H2O measurement
x:Assuming that H2O vol % in a mixed gas is measured:
y = (100 – x) 3 0.21 …………………. Equation (2)

 Loading...
Loading...