10-16
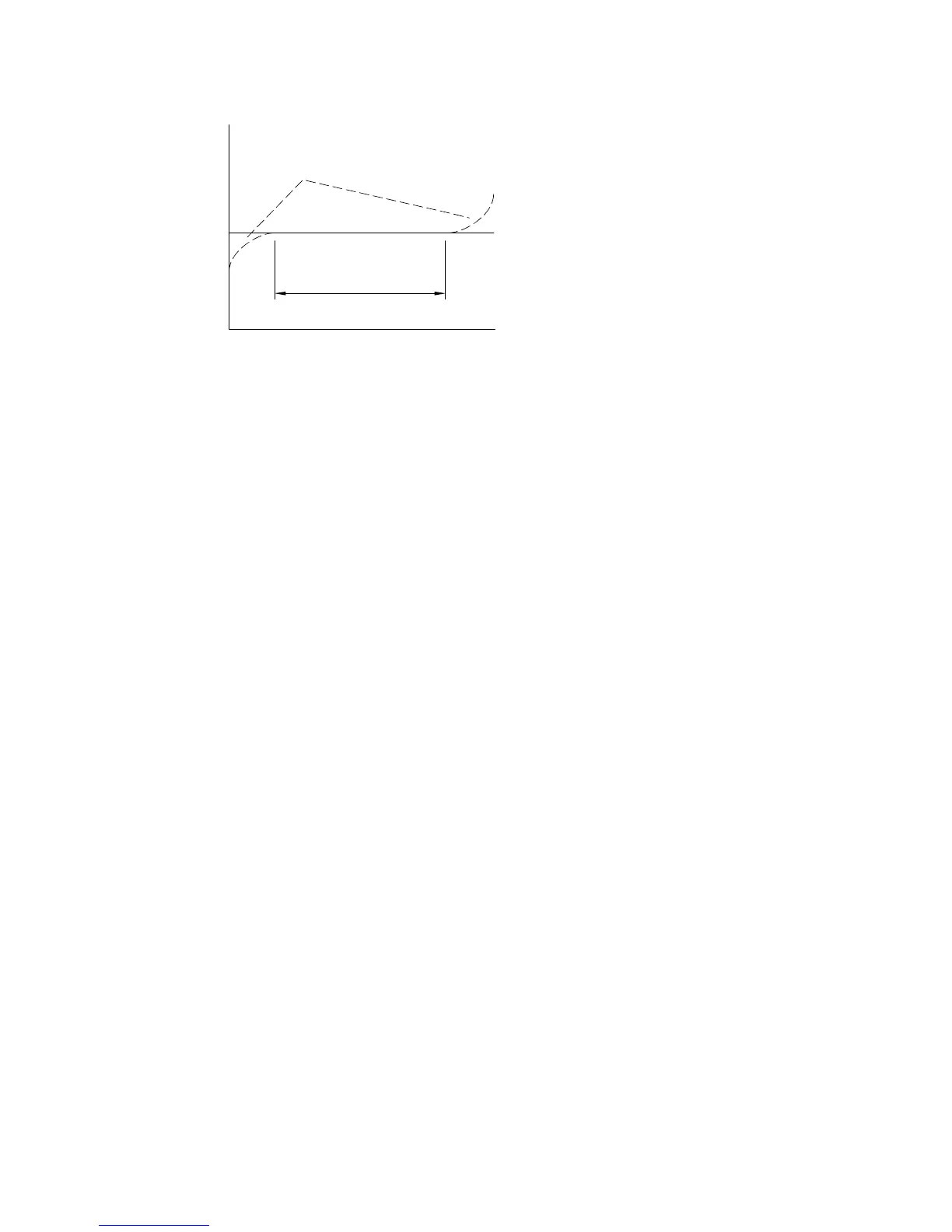
TIME WINDOW
i/t
TYPICAL DEVIATIONS
IDEAL
t
-1/2
Figure 10-8.
Plot of i/t
-1/2
vs. t for
CA
showing "time window" for condition of planar diffusion.
Although
CA
and
CC
are not used to measure absolute concentrations, they can be
used to measure changes in concentrations due to homogeneous chemical reactions of
the electrolyzed molecules (3,4). This uses the double step technique, and measures
the ratio of the forward and backward currents (or charges). If the molecules undergo
a chemical reaction after electrolysis in the forward step, then these molecules are not
available for electrolysis in the reverse step. Hence, the faster the chemical reaction,
the smaller the current/charge on the reverse step. The rate of the chemical reaction
can be calculated by measuring the current (or charge) ratio at different pulse widths.
If the final potential is at a value at which the electron transfer does not occur rapidly,
then the current (or charge) response may be influenced by the rate of heterogeneous
electron transfer as well as the rate of diffusion. The rate of electron transfer can
therefore be measured by
CA
and
CC
(5).
CC
has several advantages over
CA
. The signal increases with time, so the latter
parts of the response, which are least distorted by the finite charging time of the
electrode, offer better signal-to-noise ratios (in addition, any white noise tends to
averaged by integration) . In addition, since charge is a summation over the time
frame of the experiment, the information from the early response is retained.
However, in some instruments, this is not strictly the case, since the charge is
obtained by integrating the discrete current values, and some charge is invariably lost.
In contrast, in the BAS 100B/W, there is a 'true' charge-to-voltage converter, which
means that none of the early information is lost.
Another application of
CC
which takes advantage of it's ability to retain early
information is the detection of species adsorbed to the surface of the working
electrode (3). Such species are electrolyzed very rapidly when the potential is
stepped. The total charge measured during the
CC
experiment is
Q = Q
diff
+ Q
dl
+ Q
ads
 Loading...
Loading...