14 Instructions for use Flowmeter
English / English USEnglish / English US
Fault-Cause-Remedy
Maintenance
This chapter describes the maintenance measures
required to maintain the proper functioning of the
medical device. Maintenance measures must be
performed by the personnel responsible.
Definition of maintenance concepts
Inspection
Perform inspections at regular intervals and observe
the following specifications.
.
Performing inspection
1 Check completeness and readability of labels
2 Visual inspection of
– connectors
– gas type code conformity
– the sealing ring at the gas outlet
– the CS connection hose, if available
– the pressure dome and the respective seal-
ing ring for cracks and contamination
3 Functional check
Repair
Dräger recommends that all repairs are carried out
by DrägerService and that only authentic Dräger
repair parts are used.
Cleaning and disinfection
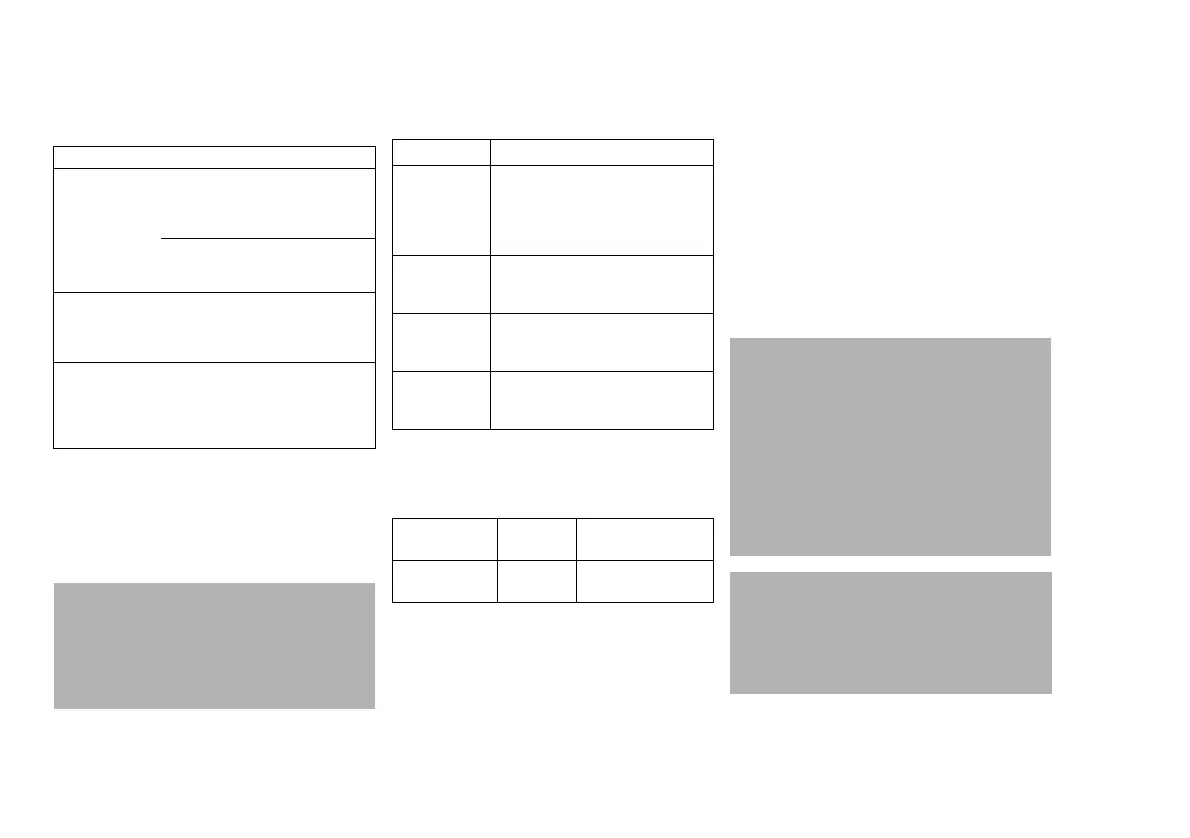
Fault Cause Remedy
Ball sticks after
flow has been
terminated
Static charge Disconnect
flowmeter and
set down
briefly
Soiling inside
the flow tube
Replace flow
tube, call
DrägerService
Cracks and/or
clouding of the
pressure dome
Incorrect
disinfectant or
exposure to
sunlight
Replace the
pressure
dome. Call
DrägerService
Leakage
between
flowmeter and
connected
accessories
Missing or
worn sealing
ring at the gas
outlet
Call
DrägerService
WARNING
Risk of infection
Users and service personnel can become
infected with pathogenic germs.
Disinfect and clean device or device parts
before any maintenance measures and also
before returning the medical device for repair.
Concept Definition
Maintenance All measures (inspection,
preventive maintenance, repair)
intended to maintain and restore
the functional condition of a
medical device
Inspection Measures intended to determine
and assess the actual state of a
medical device
Preventive
maintenance
Recurrent specified measures
intended to maintain the functional
condition of a medical device
Repair Measures intended to restore the
functional condition of a medical
device after a device malfunction
Checks Interval Personnel
responsible
Inspection Every
5years
Service personnel
WARNING
Risk of infection
Reusable products must be reprocessed. Oth-
erwise there is an increased risk of infection
and the functioning of the products may be im-
paired.
– Comply with the hospital’s hygiene regula-
tions.
– Use validated procedures for reprocessing.
– Reprocess reusable products before each
use.
– Observe the manufacturer’s instructions on
cleaning agents and disinfectants.
WARNING
Risk due to faulty products
Reusable products may show signs of wear,
such as cracks, deformations, discolorations,
or peeling.
Check products for signs of wear and replace if
necessary.
 Loading...
Loading...











