98
Recommended separation distances between
Portable and mobile RF communications equipment and the TU101
The TU101 is intended for use in the Electromagnetic environment in which radiated RF disturbances
are controlled. The customer or the user of the TU101 can help prevent electromagnetic interference
by maintaining a minimum distance between portable and mobile RF communications equipment
(transmitters) and the TU101 as recommended bellow, according to the maximum output power of the
communications equipment.
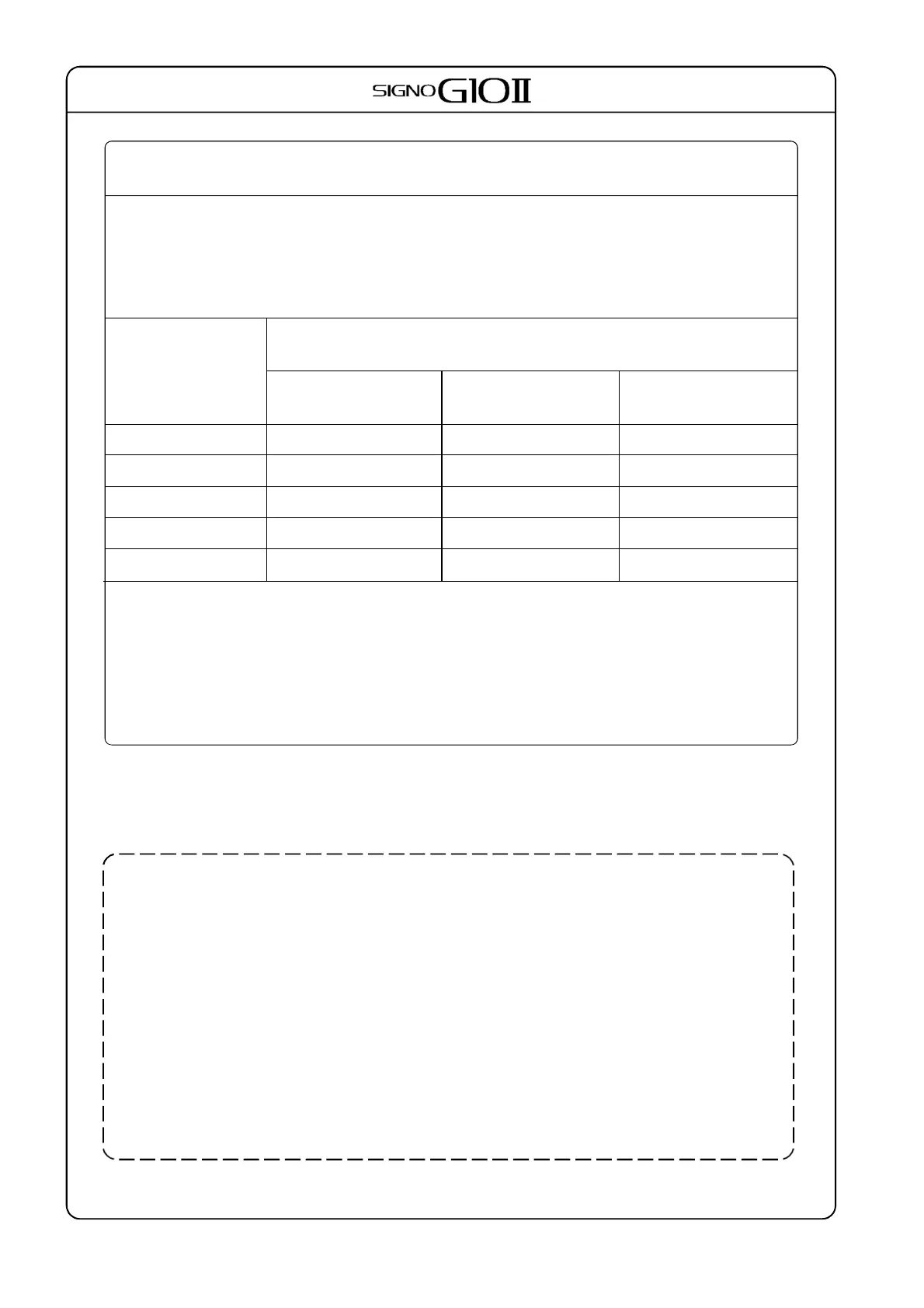
Rated maximum
output power of
transmitter
[W]
Separation distance according to frequency of transmitter
[m]
150KHz to 80MHz
d = 1.17 ×√ P
80MHz to 800MHz
d = 1.17 ×√ P
800MHz to 2.7GHz
d = 2.33 ×√ P
0.01 0.12 0.12 0.23
0.1 0.37 0.37 0.74
1
1.2 1.2 2.3
10 3.7 3.7 7.4
100 3.7 3.7 7.4
For transmitters rated at a maximum output power not listed above, the recommended separation dista-
nce d in meter(m) can be estimated using the equation applicable to frequency of the transmitter,
where P is the maximum output power rating of the transmitter in watts(W) according to the transmi-
tter manufacturer.
NOTE 1: At 80MHz and 800MHz the separation distance for the higherfrequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures objects and people.
System sentence
Additional equipment connected to medical electrical equipment must comply with the respective
IEC or ISO standards(e.g. IEC 60950 for data processing equipment). Furthermore all
configurations shall comply with the requirements for medical electrical systems (see IEC 60601-1-
1 or clause 16 of the 3Ed. of IEC 60601-1,respectively).
Anybody connecting additional equipment to medical electrical equipment configures a medical
system and is therefore responsible that the system complies with the requirements for medical
electrical systems. Attention is drawn to the fact that local laws take priority over the above
mentioned requirements. If in doubt, consult your local representative or the technical service
department.
Sources:
-IEC 60601-1:2005:7.9.2.5,8.1,16.2.d
-MDD 93/42/EEC: Annex I clause 13.6.c.
 Loading...
Loading...