12
Dissolved Oxygen Theory
35
Dissolved Oxygen (DO) refers to the volume of oxygen that is contained in water.
There are two main sources of DO in water; atmosphere and photosynthesis.
Waves and tumbling water mix air into the water where oxygen readily
dissolves until saturation occurs. Oxygen is also produced by aquatic plants
and algae during photosynthesis.
The amount of DO that can be held by water depends on 3 factors:
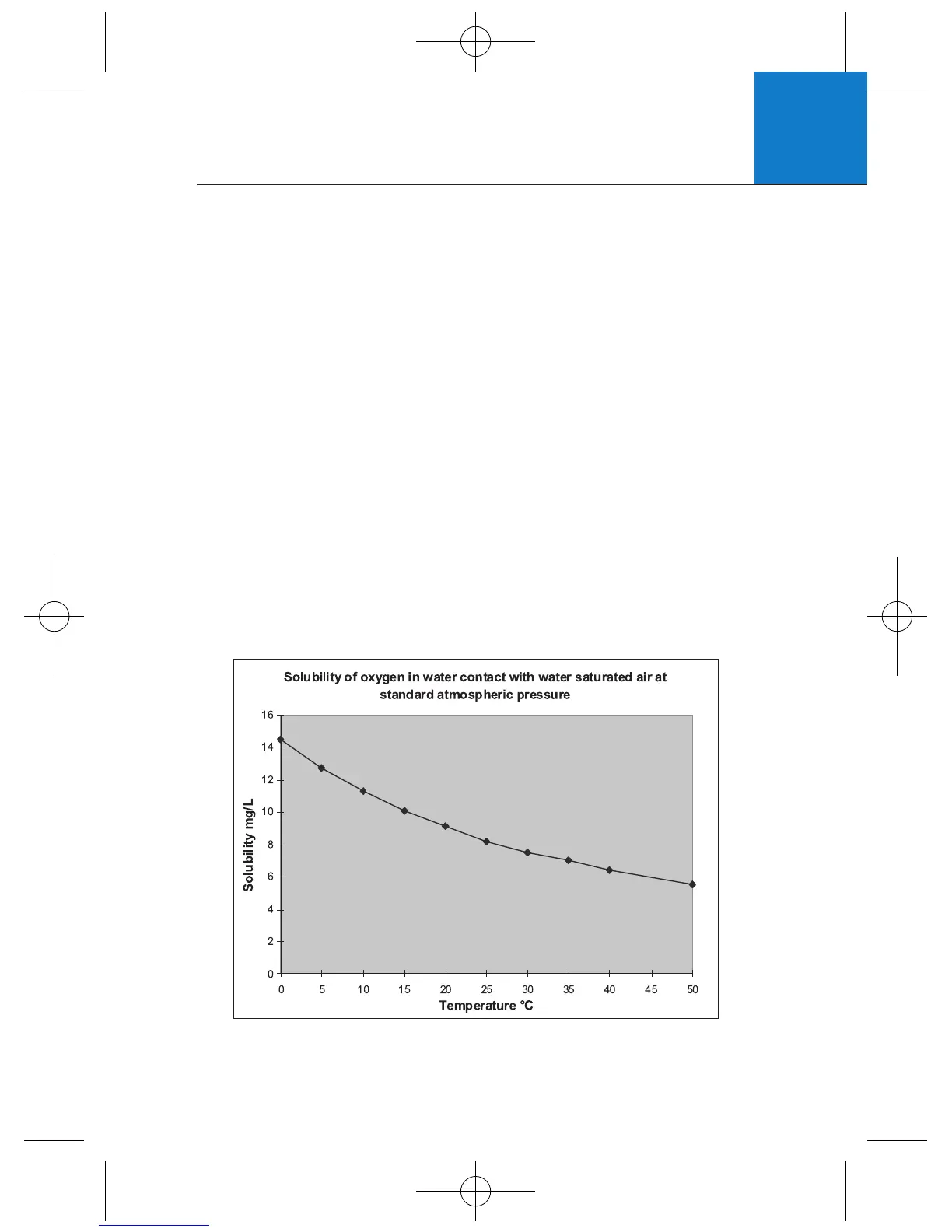
1 TEMPERATURE:
DO increases with decreasing temperature
(colder water holds more oxygen)
2 SALINITY:
DO increases with decreasing salinity
(freshwater holds more oxygen than saltwater does)
3 ATMOSPHERIC PRESSURE:
DO decreases with decreasing atmospheric pressure
(amount of DO absorbed in water decreases as altitude increases)
12.0 Dissolved Oxygen Theory
DO Solubility in Water vs Temperature
 Loading...
Loading...








