38
Note: It is understood that all items in the following table will be used as intended, and the level of
disinfection or sterilization required may vary according to local regulations.
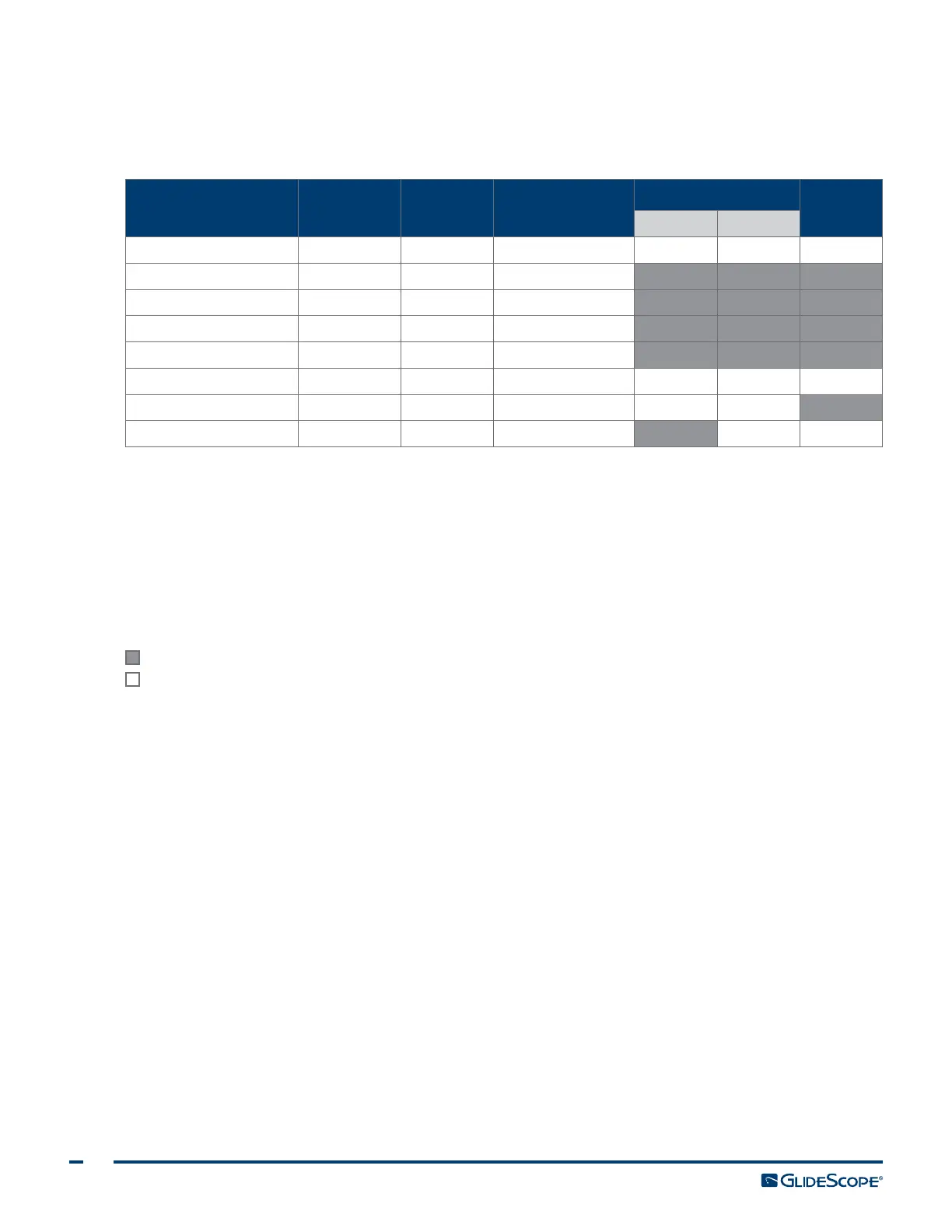
Table 5. System Risk Classification
DEVICE PACKAGED USE
SPAULDING’S/CDC
CLASSIFICATION
DISINFECTION LEVEL
STERILIZE
Low High
Smart Cable Nonsterile Reusable Noncritical X
||
Monitor* Nonsterile Reusable Noncritical
Cradle* Nonsterile Reusable Noncritical
Cart* Nonsterile Reusable Noncritical
GVL
®
Stat
†
Sterile Single‑Use Semi‑critical
Video baton
‡
Nonsterile Reusable Noncritical X
||
GlideScope Direct Nonsterile Reusable Semi‑critical X
GlideRite
®
Rigid Stylet
§
Nonsterile Reusable Semi‑critical X
* Clean the video monitor, cradle, and cart when they are visibly soiled and on a regular basis, as per a schedule established by the medical
care facility or provider.
† Single‑use Stats may not be cleaned, disinfected, or sterilized. Dispose of single‑use Stats after use.
‡ The video baton is a nonsterile, reusable device, which is protected from contact with mucous membranes and non‑intact skin by the
Stat (sterile, single‑use) when used as intended. Low‑level disinfection is recommended for the video baton after every patient use.
High‑level disinfection is required for the video baton when it is visibly soiled.
§ For instructions on cleaning and disinfection, see the GlideRite Rigid Stylet Operations and Maintenance Manual.
|| The low‑level disinfection solutions in this manual are not available in all geographic regions. If they are not available in your region, such
as the United States, use a high‑level disinfection method only.
X Checked boxes show minimum disinfection level requirement.
Shaded areas indicate that the disinfection/sterilization level is not required or not compatible with the device materials.
Unshaded areas show permissible levels of disinfection or sterilization based on compatibility with the device materials.
 Loading...
Loading...