Density Meter LB 444 SECTION 6. BASICS OF DENSITY MEASUREMENTS
73
Calculating the Density of Individual Components
To calculate the concentration of suspensions and solutions from the measured
density value, you need to know the density of both components and enter it as
Solids density and Liquid density in the product parameters.
With suspensions the solids density is usually known and water is used as car-
rier liquid (select Water TC and enter the solids density); with solutions, on the
other hand, the density of the relevant components often has to be calculated
from table values.
Table 1 in the Appendix lists the values for common products. If your product is
not included, just use the formula below for calculation.
Using the values listed in the table, you can calculate the density of the compo-
nents. Enter the density of the carrier liquid or the density of the attendant com-
ponent (mostly water) at average temperature (reference temperature) as liquid
density.
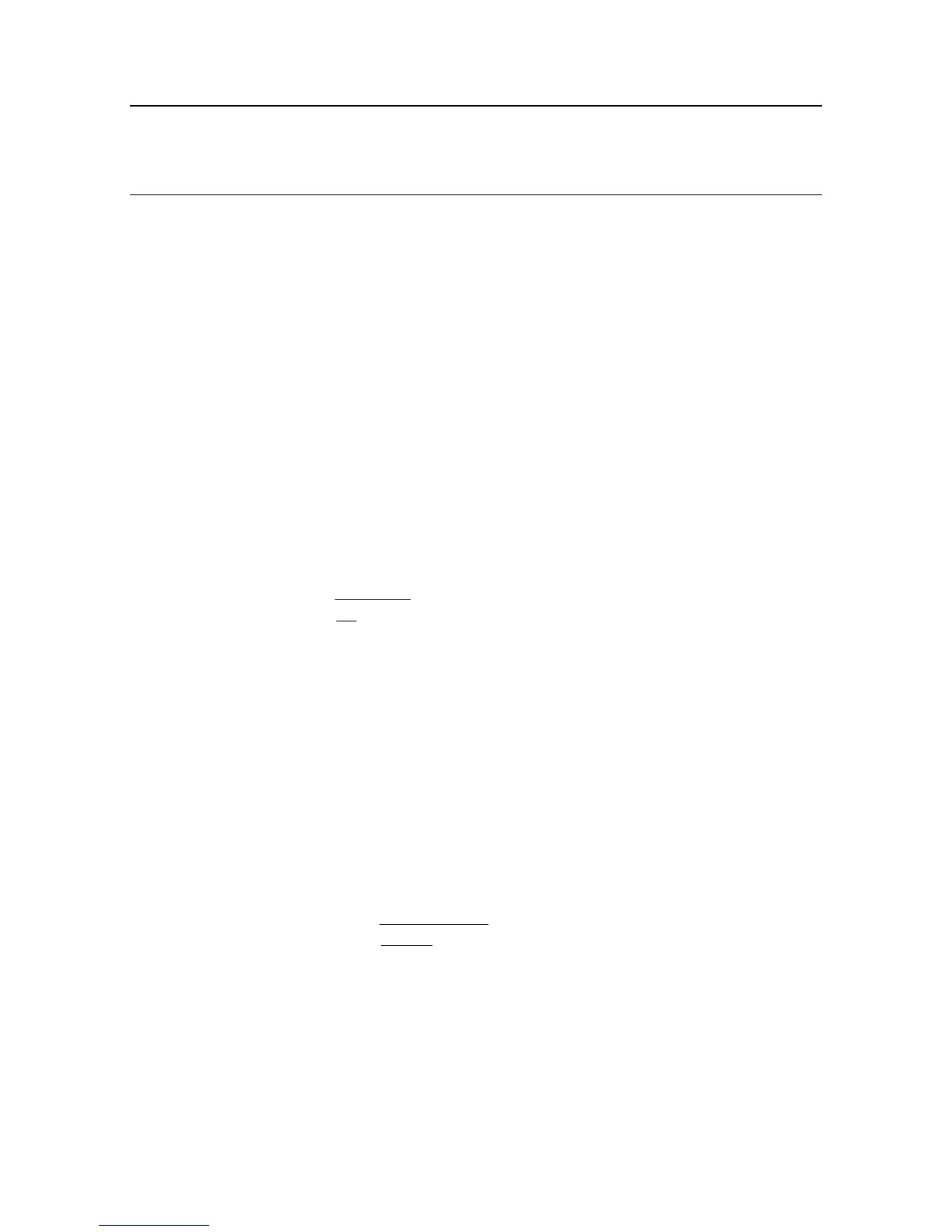
a) Table values are available as % concentration/density
ρ
ρ
ρ
ρ
S
L
L
C
C
=
−+
,
,
*
1
C’ = Concentration in weight percent / 100 (for example, at 20% C’ = 0.2)
ρ
L
= Density of the attendant component in g/cm
3
(liquid density)
ρ = Density of mixture in g/cm
3
at average concentration
ρ
S
= Density of the component to be measured (solids density)
Example:
Product HCl - H
2
O
Measuring range: Concentration 10 - 30% HCl
Average temperature 20°C
Density ρ at 20°C and 20% concentration: 1.0980 g/cm
3
Density H
2
O (ρ
L
) at 20°C: 0.99823 g/cm
3
ρ
S
=
−+
=
02 099823
099823
10980
102
18294
.*.
.
.
.
.
Input liquid density: 0.99823
Input solids density: 1.8294
 Loading...
Loading...