19
217
Eversense E3 CGM User Guide
217
Eversense E3 CGM User Guide
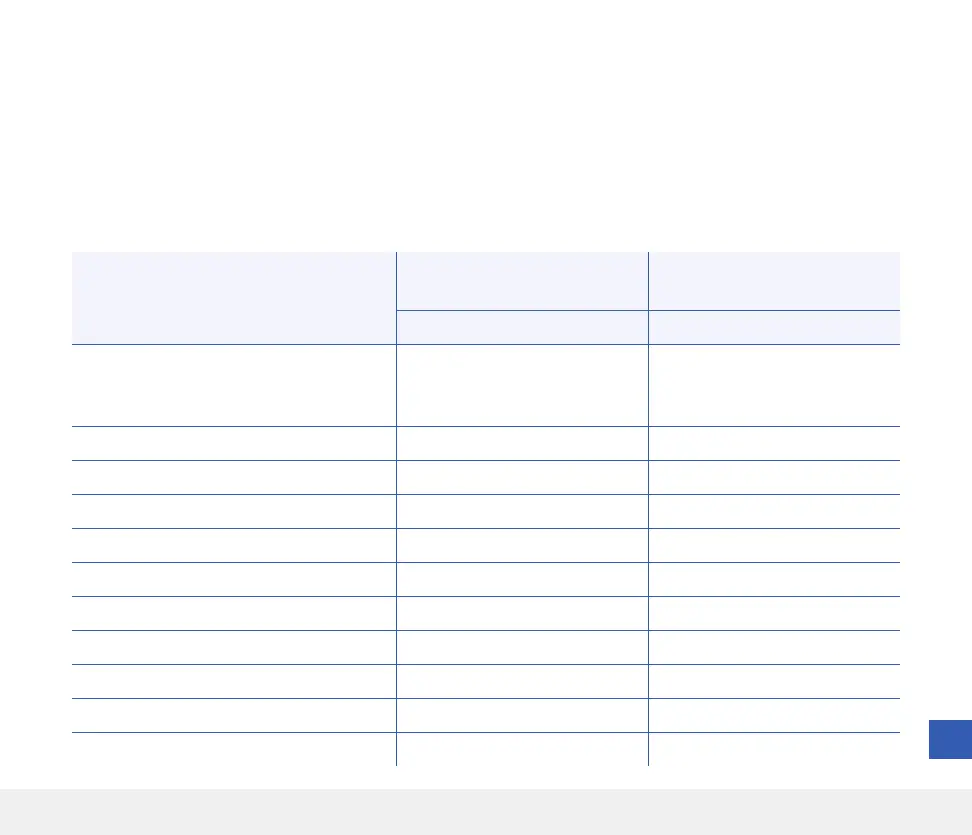
Number of Events
Number of Subjects
(% of Subjects)
Event Type 59 37 (20.4)
Skin irritation, adhesive patch location or
insertion site (including erythema, pruritus,
rash, contact dermatitis, seroma)
16 11 (6.1)
Skin atrophy 4 4 (2.2)
Hypopigmentation 4 3 (1.7)
Infection (procedure related) 2 2 (1.1)
Infection (not procedure related) 1 1 (0.6)
Bruising 19 11 (6.1)
Bleeding 3 3 (1.7)
Pain 7 6 (3.3)
Arm Numbness 1 1 (0.6)
Tremor 1 1 (0.6)
Adhesive Skin Closure Strips did not hold 1 1 (0.6)
Table 11 – Adverse Events (All Subjects, n = 181)
Safety
The PROMISE study lasted for 180 days, and the number of related adverse events was recorded. The Eversense E3
CGM System was well tolerated in the study. During the study's 31,373 sensor wear days, there were no unanticipated
adverse events. Fifty-nine adverse events were reported in 37 participants. None of the adverse events resulted in
hospitalisation.
 Loading...
Loading...