30 HVAD® Instructions for Use
10
9
8
7
6
5
4
3
2
1
Appendix
Reference
Guides
Alarms and
Emergencies
Patient
Management
Surgical
Implant and
Explant
Monitor
Peripherals
and
Accessories
HVAD
®
Pump Overview
Introduction
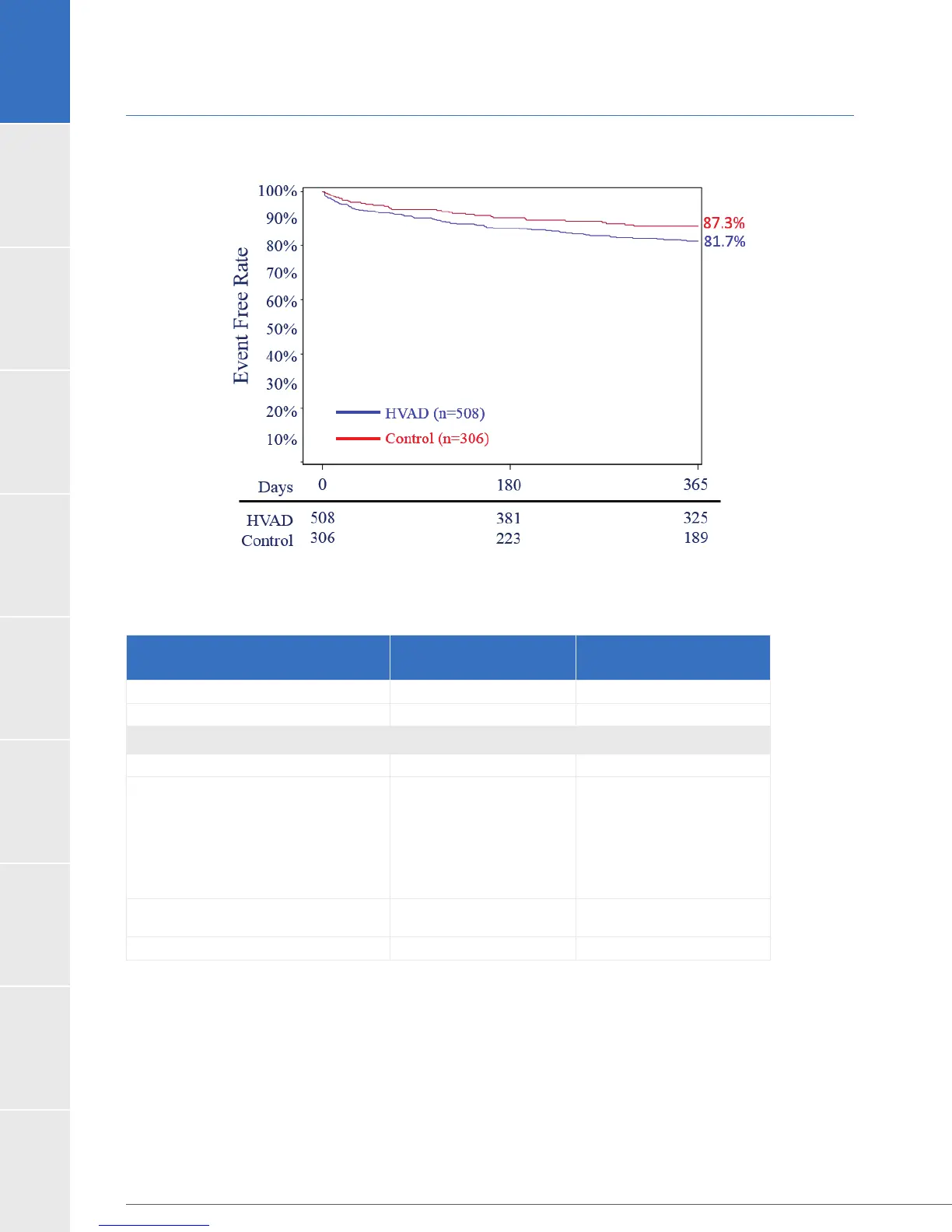
1.8 US Clinical Study: Destination Therapy (continued)
B) Survival on Original Device Free from Neurologic Events (Strokes with mRS>0, TIA or SCI)
Table 14: Binary Analysis of ENDURANCE Expanded Dataset: Survival at 2 years free from
disabling stroke (mRS ≥4) and alive on the originally implanted device, or transplanted
or explanted for recovery.
Event Free Survival at 2 years
HVAD
Control
Success 55.2% (164) 57.4% (85)
Failure 44.8% (133) 42.6% (63)
If Failure, reason:
Patient dies 34.7% (103) 26.4% (39)
Device malfunction or failure
requiring exchange, explant or
urgent transplant
Exchange
Explant
Urgent Transplant
8.8% (26)
7.7% (23)
0.0% (0)
1.0% (3)
16.2% (24)
13.5% (20)
0.7% (1)
2.0% (3)
Disabling stroke
1.0% (3) 0
Imputed failure* 0.3% (1) 0
*Patient experienced a stroke prior to their 2-year endpoint, and died beyond the 2 year endpoint, but
before the 24 week MRS assessment.
 Loading...
Loading...