2 HVAD® Instructions for Use
10
9
8
7
6
5
4
3
2
1
Appendix
Reference
Guides
Alarms and
Emergencies
Patient
Management
Surgical
Implant and
Explant
Monitor
Peripherals
and
Accessories
HVAD
®
Pump Overview
Introduction
1.0 Introduction
In this manual, there will be the following symbols:
1.1 Introduction
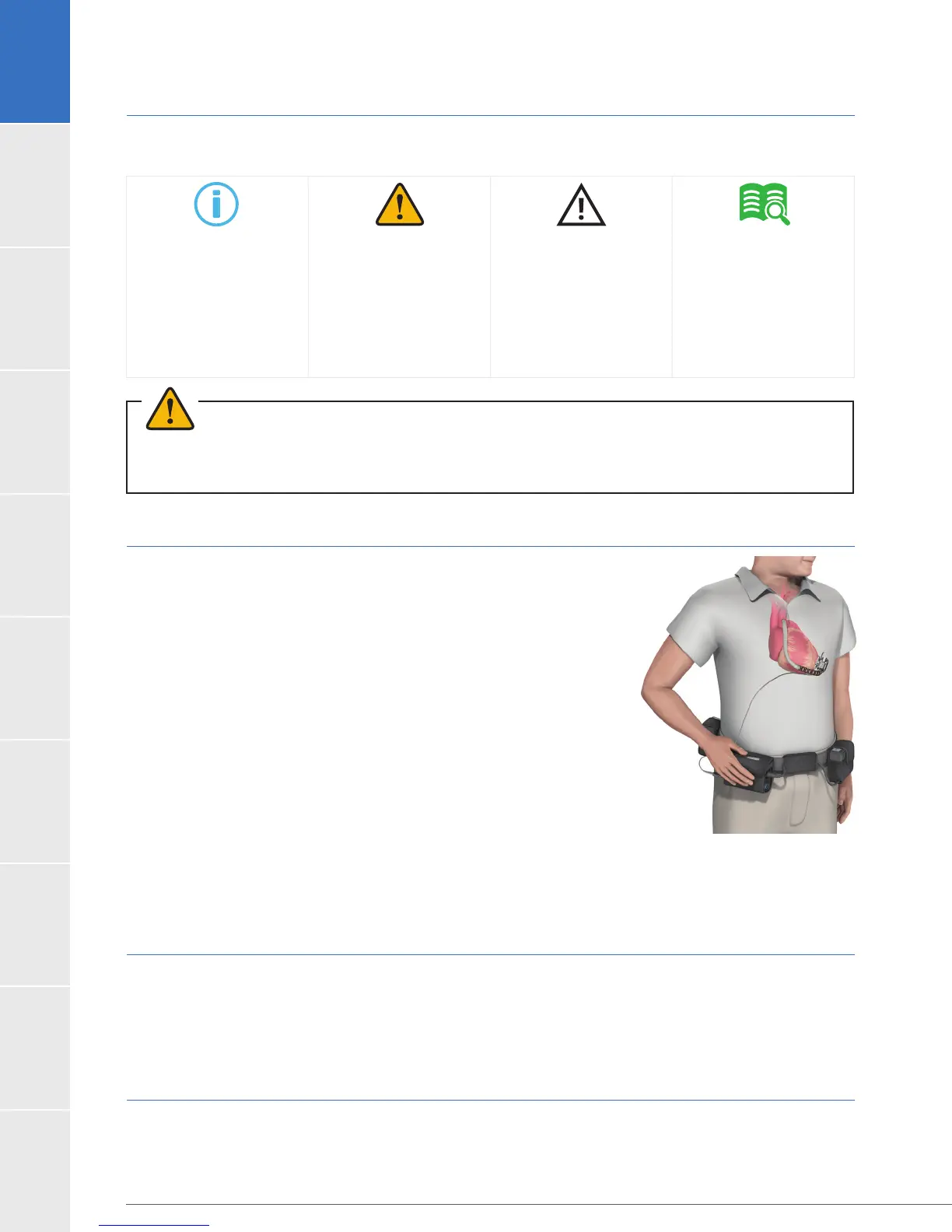
The HeartWare
™
HVAD
™
System is designed to assist a weakened, poorly
functioning left ventricle. The HeartWare
™
HVAD
™
System is designed
for in-hospital and out-of-hospital settings, including transportation via
™
HVAD
™
System utilizes
a centrifugal blood pump, the HVAD
®
Pump (the “pump”), which is
implanted in the pericardial space with left ventricular apex to
conduit, which is partially sintered, is integrated with the pump and
attached to the pump. A percutaneous driveline connects the
pump to an external controller. The controller, powered by two
batteries or by one battery and electricity from a wall or car outlet,
regulates pump function and monitors the system. The monitor is used to
display system performance and to change controller operating
parameters. A battery charger is also included.
All components of the HeartWare
™
HVAD
™
System are designed to be
used only in conjunction with each other. They are neither compatible
nor intended to be used with other manufacturer’s devices.
1.2 Indications for Use
The HeartWare
™
HVAD
™
System is indicated for hemodynamic support in patients with
advanced, refractory left ventricular heart failure; either as a Bridge to Cardiac Transplantation
(BTT), myocardial recovery, or as Destination Therapy (DT) in patients for whom subsequent
transplantation is not planned.
1.3 Contraindications
The HeartWare
™
HVAD
™
System is contraindicated in patients who cannot tolerate
anticoagulation therapy.
Figure 1:
HeartWare
™
HVAD
™
System
Indicates there is more
information available in the
manual and the location in
the manual
as a Warning
A Warning is a statement
about the possibility of
injury, death or other serious
adverse reaction that is
associated with the use or
misuse of the device
as a Caution or Precaution
A Caution is a statement
that not following the
instruction may lead to
device misuse, malfunction
or damage
Indicates there is
a quick reference
guide available
WARNING! Carefully read this entire manual prior to implanting or operating the device. Improper
operation of the system and potential harm to the patient and to the user could result.

 Loading...
Loading...