10
9
8
7
6
5
4
3
2
1
Appendix
Reference
Guides
Alarms and
Emergencies
Patient
Management
Surgical
Implant and
Explant
Monitor
Peripherals
and
Accessories
HVAD
®
PumpOverviewIntroduction
11Introduction
1.7 Pivotal US Clinical Study: Bridge-to-Transplant
Pivotal Clinical Study Design
This was a multi-center, prospective, contemporaneous control trial. The trial was non-randomized
and open label. Enrollment in the study is complete, subjects have all reached the primary
Subjects were consented for participation and then assessed against the inclusion and
exclusion criteria for participation in the study and implantation of the HVAD
®
Pump. After the
surgical recovery period, patients were allowed to leave the hospital if they met additional
criteria for hospital discharge. Each patient was followed to 180 days, death, device explant
Patient outcomes were compared to a contemporaneously treated cohort of patients as
met the control group inclusion and exclusion criteria comprised the control group.
Study Objectives
Primary Objective
The purpose of the HeartWare
™
HVAD
™
System study was to evaluate the safety and
effectiveness of the HeartWare
™
HVAD
™
System in patients listed for cardiac transplantation with
refractory, advanced heart failure at risk of death. The primary endpoint is success at 180 days
for recovery. If explanted for recovery patients must have survived 60 days post-explant to be
considered successful.
Effectiveness was measured by the primary endpoint. The proportion of study patients alive,
transplanted, or explanted for recovery at 180 days was compared to the same proportion
Secondary Objectives including Safety
Secondary endpoints included: overall survival; incidence of all serious adverse events, including
neurocognitive status and unanticipated adverse device effects; incidence of all device
failures and device malfunctions; Quality of Life improvement, as measured by the Kansas City
Cardiomyopathy Questionnaire (KCCQ) and European Quality of Life Assessment (EuroQol)
Safety measures included the frequency and rates of adverse events, overall and for each
™
HVAD
™
System support.
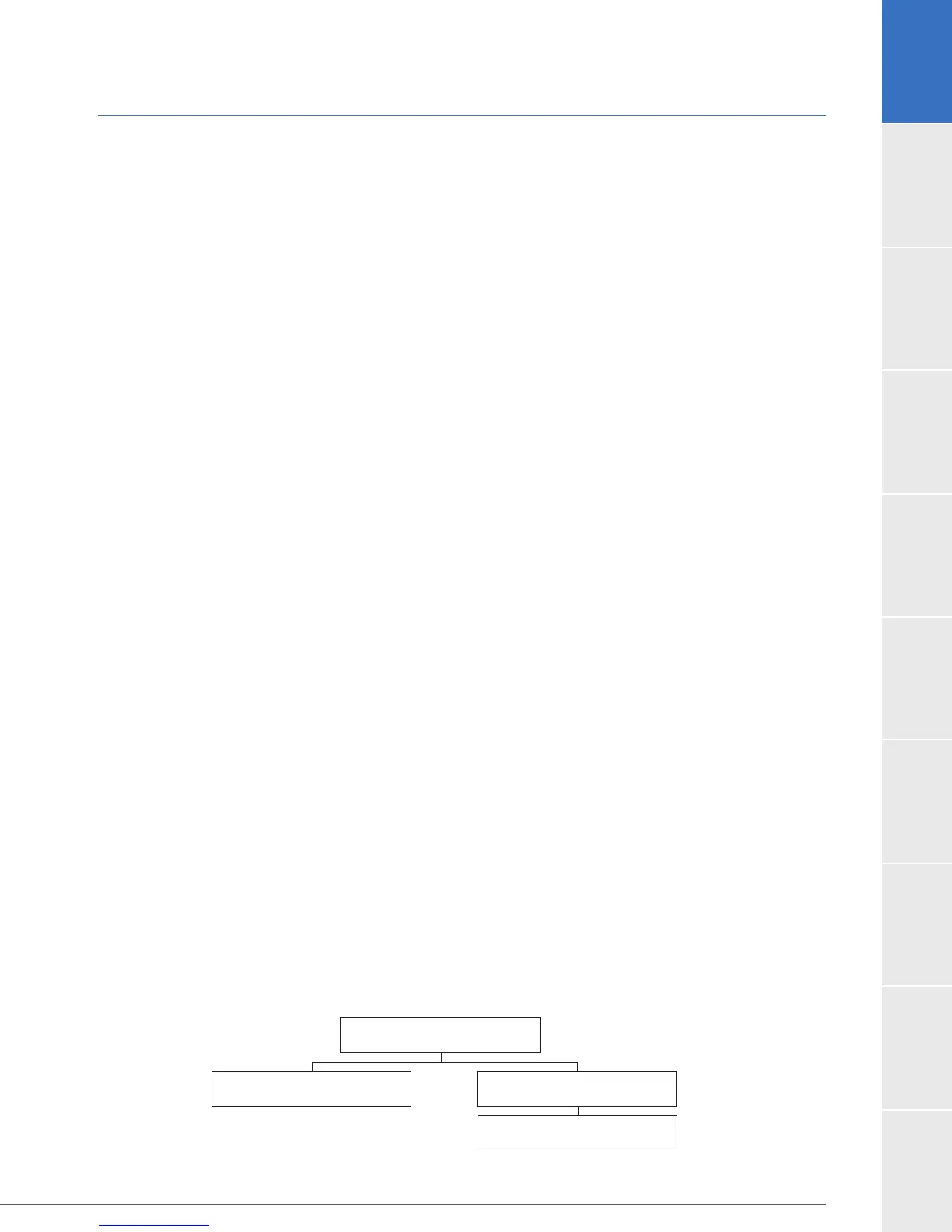
Study Population Demographics and Baseline Parameters
population, (ITT), the Safety population (SAF) and the Per Protocol population (PP).
Intent to Treat Population
N=140
Safety Population
N=140
Per Protocol Population
N=137
Major Protocol Violations
N=3
 Loading...
Loading...