10
9
8
7
6
5
4
3
2
1
Appendix
Reference
Guides
Alarms and
Emergencies
Patient
Management
Surgical
Implant and
Explant
Monitor
Peripherals
and
Accessories
HVAD
®
PumpOverviewIntroduction
1.0 Introduction
Introduction
1.1 Introduction .................. 2
1.2 Indications for Use ....... 2
1.3 Contraindications .........2
1.4 Warnings ......................3
1.5 Precautions ..................7
1.6 Potential
Complications
............... 10
1.7 Pivotal US Clinical Study:
Bridge-to-Transplant
..11
1.8 US Clinical Study:
Destination Therapy
....20
1.9 Destination Therapy
Supplemental Study
...38
Foreword
The HeartWare
™
HVAD
™
System is indicated for use
under the direct supervision of a licensed healthcare
practitioner or by personnel trained in its proper use.
Clinical users include physicians, registered nurses,
perfusionists and biomedical engineers. Implant of
surgeon trained by HeartWare-authorized personnel.
Clinical users of the HeartWare
™
HVAD
™
System should
attend HeartWare training, should have a working
knowledge of the principles of ventricular assist devices
(VADs), and should be aware of the physical and
psychological needs of patients undergoing VAD
support. Patients and caregivers should complete a user
training program and demonstrate their ability to use the
system prior to independence.
Clinicians should read the entire Instructions for Use
before system operation. This manual may serve
as a reference for detailed information including
implant and maintenance. This manual is not intended
to replace comprehensive educational programs
or to supersede acquired knowledge or proper
medical judgment.
1
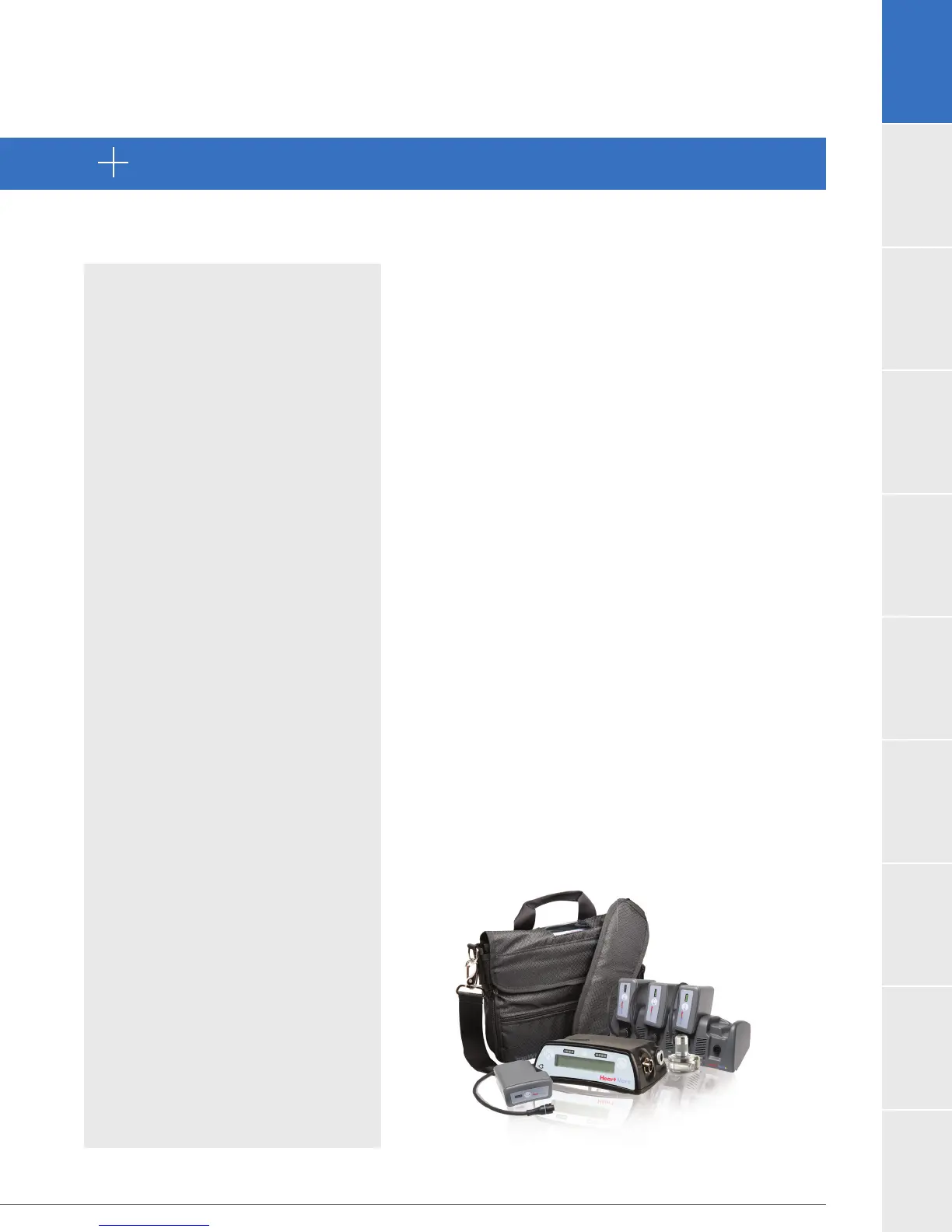 Loading...
Loading...