10
9
8
7
6
5
4
3
2
1
Appendix
Reference
Guides
Alarms and
Emergencies
Patient
Management
Surgical
Implant and
Explant
Monitor
Peripherals
and
Accessories
HVAD
®
PumpOverviewIntroduction
39Introduction
1.9 Destination Therapy Supplemental Study (continued)
primary endpoint, in that it compared the composite of stroke-free (mRS < 4 at 24 weeks
post-stroke) survival while on the original device between HVAD and Control arms;
endpoint. This endpoint was to test for non-inferiority of the HVAD to the control device, with
a non-inferiority margin of 15%.
Additional endpoints included adverse events, device malfunctions and failures, as well as
health status and functional improvements.
B. Accountability of PMA Cohort
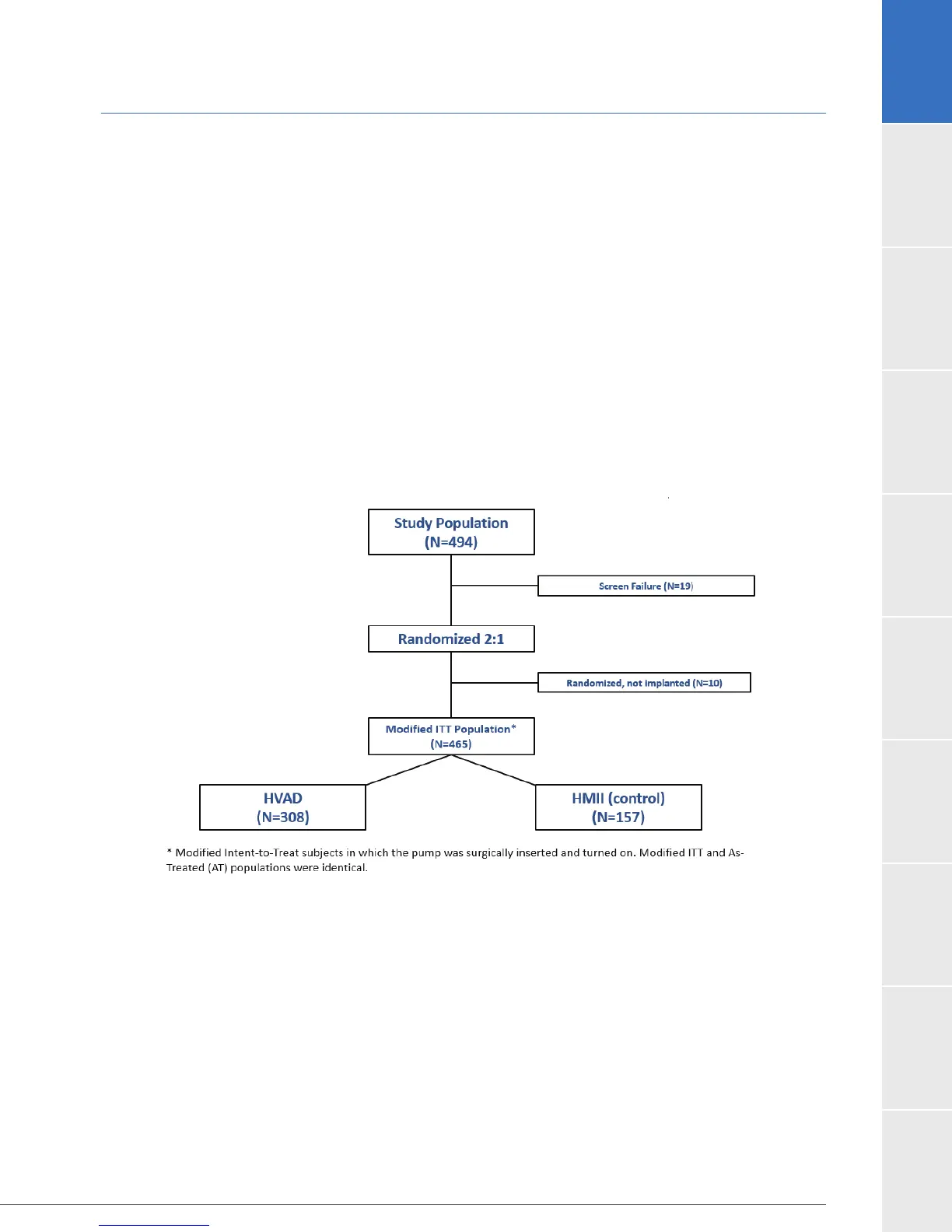
93.7% (463) patients were available for analysis of the primary objective at the completion of the
study, the 12-month post-operative visit. The disposition of the patients is shown in Figure 15.
Figure 15:
 Loading...
Loading...