10
9
8
7
6
5
4
3
2
1
Appendix
Reference
Guides
Alarms and
Emergencies
Patient
Management
Surgical
Implant and
Explant
Monitor
Peripherals
and
Accessories
HVAD
®
PumpOverviewIntroduction
41Introduction
1.9 Destination Therapy Supplemental Study (continued)
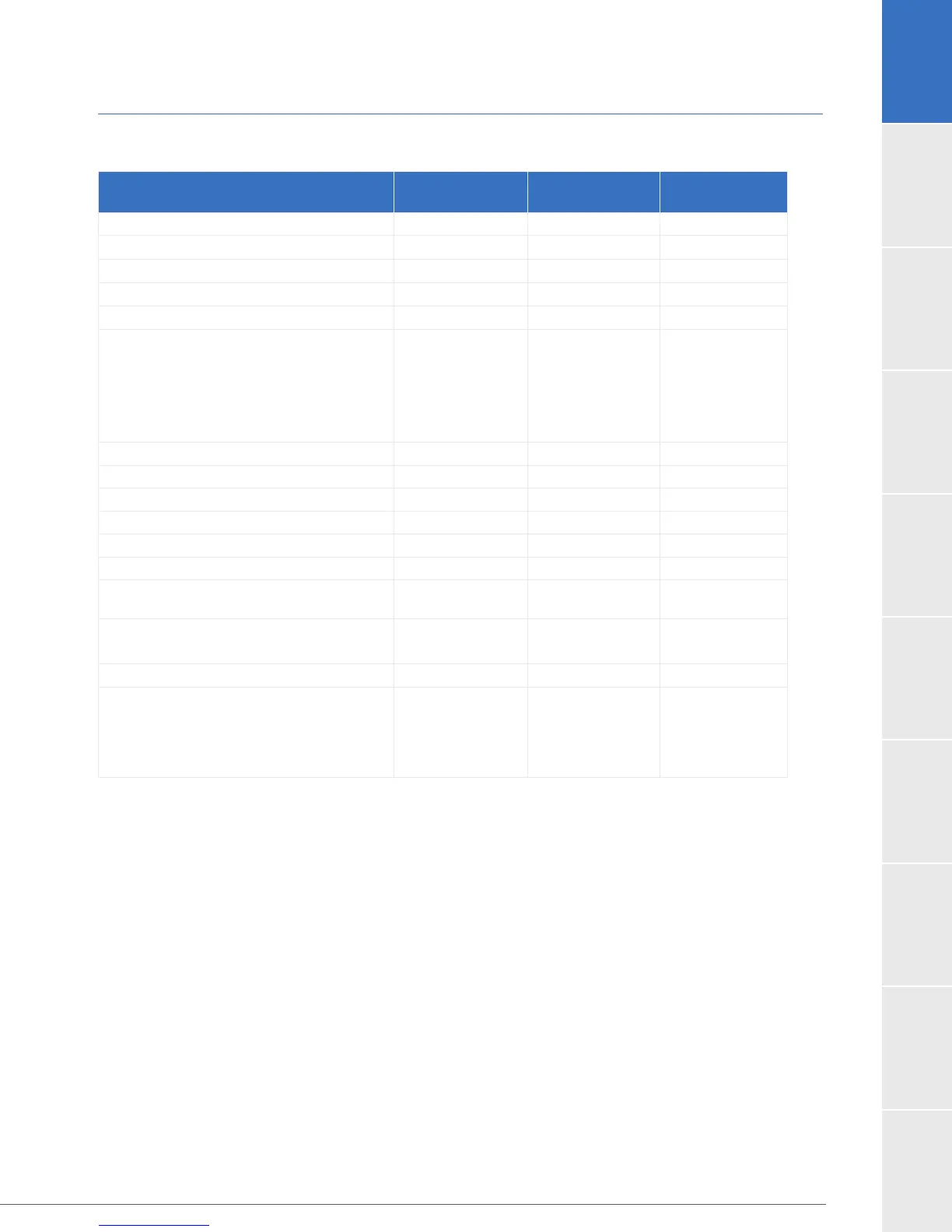
Table 18: Patient Demographics and Baseline Characteristics in the ENDURANCE Supplemental Trial.
Demographics and Baseline
Characteristics
HVAD
Control
P-value
Age (years) 63.3 ± 11.4 64.2 ± 11.1 0.39
Female gender (%) 18.2% 20.4% 0.62
Race (% White) 71.8% 75.2% 0.51
Height (cm) 175.0 ± 9.4 175.1 ± 9.8 0.91
Body Mass Index (kg/m
2
) 28.2 ± 5.5 27.4 ± 5.2 0.13
1
2
3
4-7
3.9%
32.8%
43.3%
20.0%
2.5%
32.5%
43.3%
21.7%
0.90
Ischemic Etiology of Heart Failure 55.2% 58.0% 0.62
History of smoking 68.2% 65.6% 0.60
Diabetic 49.4% 48.4% 0.92
Previous Stroke 10.4% 8.3% 0.51
Hypertension requiring medication 75.0% 72.0% 0.50
Atrial Fibrillation 50.6% 51.0%
Mean arterial blood pressure (mmHg)
78.9 ± 11.5
77.6 ± 11.1
0.23
40.4%
44.2%
0.48
Left ventricular ejection fraction (LVEF, %) 17.3 ± 5.1 18.2 ± 4.5 0.07
Previous intervention (%)
ICD
CRT
IABP
80.8%
28.9%
19.2%
82.2%
28.7%
15.9%
0.80
0.45
Abbreviations:
ventricular ejection fraction.
Note: P-values are post-hoc and are included for information purposes only.
P-values comparing categorical values are from the Fisher’s Exact Test. P-values comparing continuous
values are from a two-sample t-test.
D. Safety and Effectiveness Results
1. Primary Endpoint
The outcome and analysis of the primary endpoint are shown in Table 19 and Figure 16.
injury as compared to 12.1% of the control subjects, with a difference of 2.6% between the
trial was not met.
 Loading...
Loading...