10
9
8
7
6
5
4
3
2
1
Appendix
Reference
Guides
Alarms and
Emergencies
Patient
Management
Surgical
Implant and
Explant
Monitor
Peripherals
and
Accessories
HVAD
®
PumpOverviewIntroduction
43Introduction
1.9 Destination Therapy Supplemental Study (continued)
2. Secondary Endpoints
Because the primary endpoint was not met, the hypotheses associated with the secondary
endpoints of stroke/TIA incidence and stroke-free success rate could not be tested. Thus,
only descriptive data are presented for the two secondary endpoints.
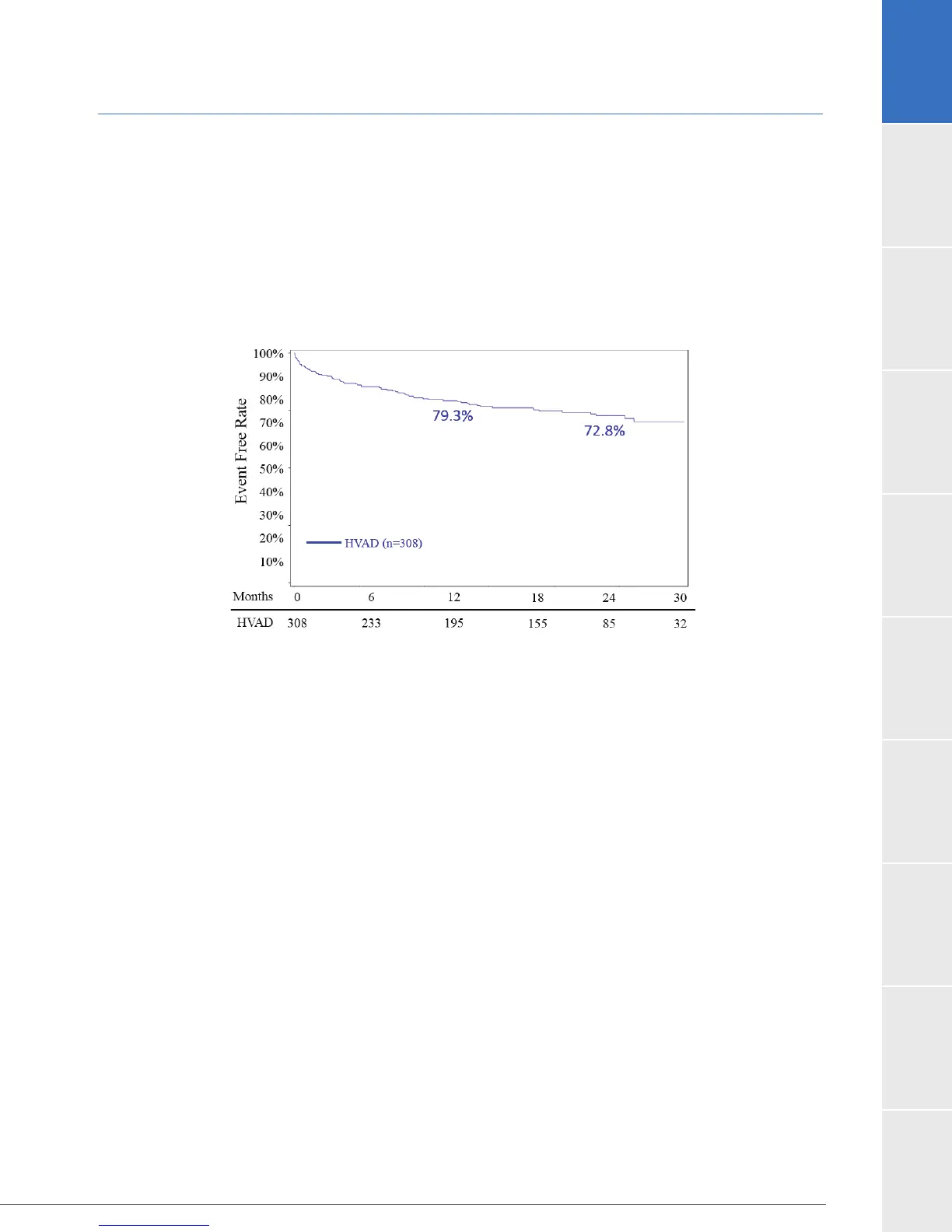
HVAD patients was 19.2% at 12 months. The Time to event curve is shown in Figure 17.
Figure 17:
The proportion of subjects who survived to one year on the original device in the absence of
HVAD arm and 66.7% in the Control arm. A freedom from event analysis is shown in Figure 17, using
data from March 27, 2017. The magnitude of the rate differential for this composite decreased
 Loading...
Loading...