79
The l
d
of the nanoparticles used for the sensor have a critical role in the sensor performance. The
l
d
determines the size of the sensing volume surrounding the nanoparticle. The electric field
intensity exponentially drops with distance from the surface of the nanoparticle so the peak
position is only sensitive to changes in RI that occur within this distance. This feature along with
the sensitivity of the nanoparticles, which is also dependent on their shape, allows Nicoya
Lifesciences Inc to tailor the performance of the sensor directly to the biomolecular system of
interest, thus maximizing performance.
7.2 Binding Kinetics
Included here is a basic overview of binding kinetics concepts. There are more thorough
reference textbooks and academic papers that can be easily accessed for more complex binding
systems - see “Recommended Reading” section.
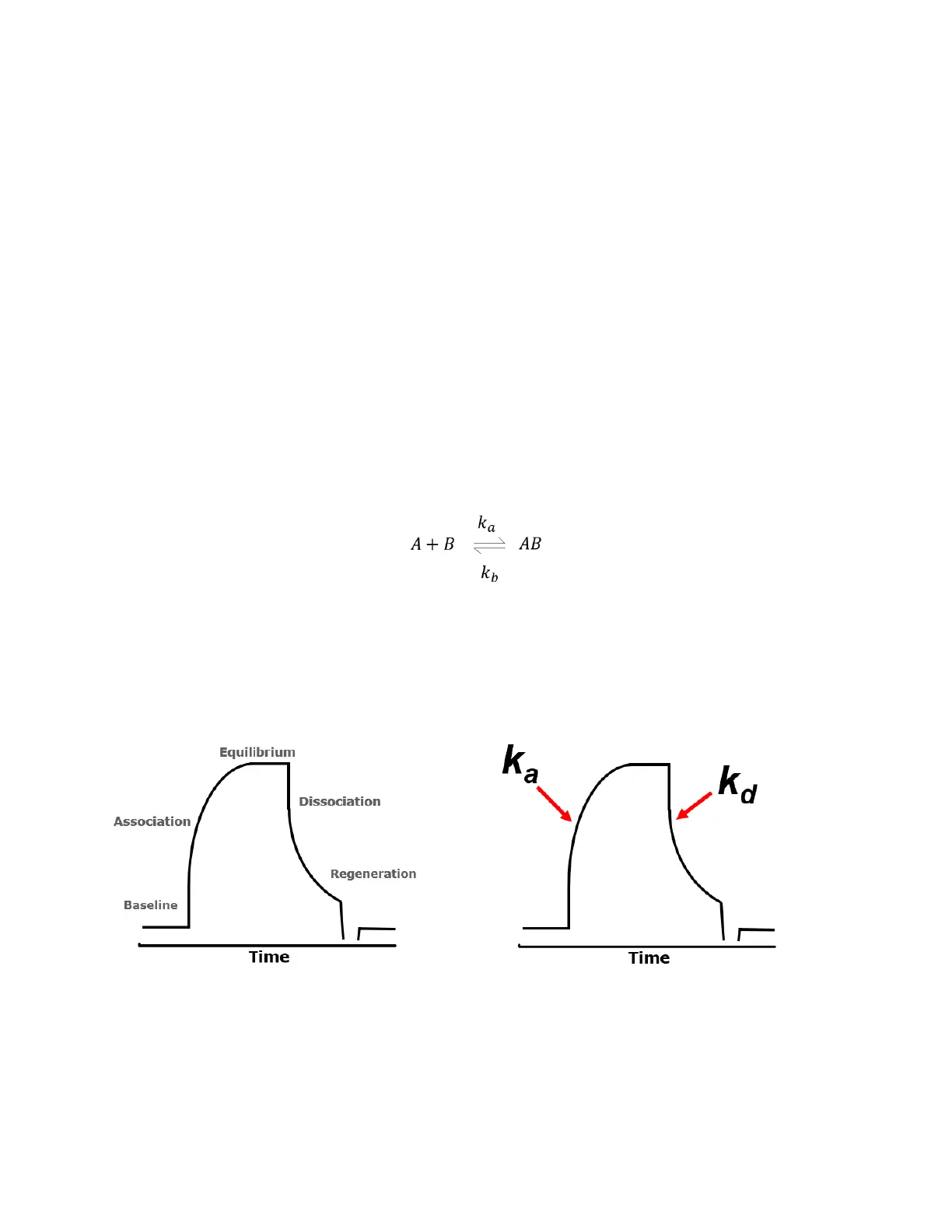
Binding kinetics describes the interaction between one or more molecular species. Interaction
includes the association and dissociation of the two species. The chemical formula describing a
simple 1:1 interaction is as follows:
Where A and B are the binding molecular species that associate to form AB. The rate of
association is governed by the rate constant k
a
and the dissociation rate by k
d
. Typical binding
curves are shown in the two graphs below. The association occurs when the analyte is in the Flow
Cell. It reaches equilibrium and then begin to dissociate once the analyte exists the Flow Cell and
is replaced by running buffer.
Figure 7.1. Typical binding curves generated for kinetic analysis
Standard rate law chemistry applies to this interaction thus at steady state the above equation
yields:
 Loading...
Loading...