2
WINGED INFUSION SET WITH FILTER
AND NEEDLE PROTECTION
Instructions for Use
ENGLISH
SYMBOL EXPLANATION
PRODUCT DESCRIPTION
Batch code
Catalogue number
Caution: Federal law (USA) restricts this device
to sale by or on the order of a physician
Consult Instructions for Use
Contents
Date of Manufacture
Do not resterilize
Do not re-use
Do not use if package is damaged and
consult instructions for use
Fragile, handle with care
Keep away from sunlight
Keep dry
Liquid lter with a pore size of 20 µm
Manufacturer
Medical Device
Non pyrogenic
Single Sterile Barrier System
Sterilized using ethylene oxide
Temperature limit
Use-by date
Rx only
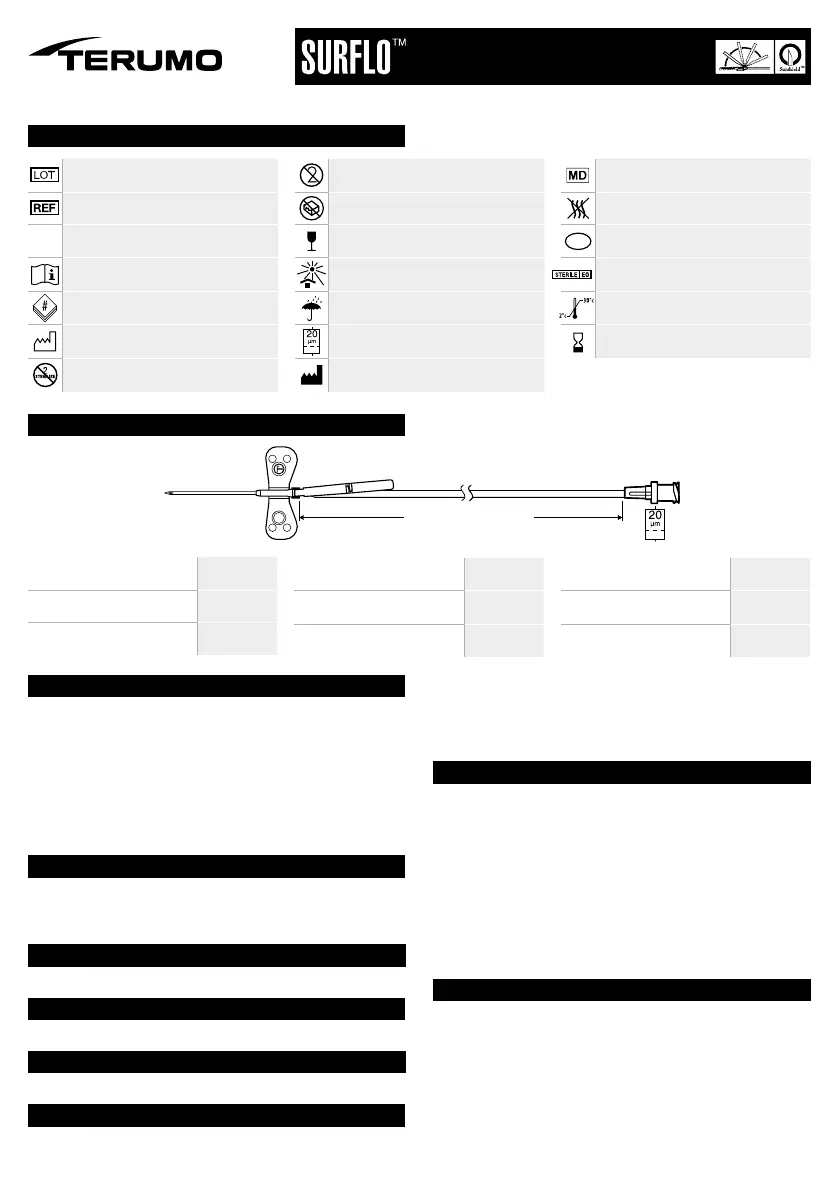
Needle Gauge (a)
23G
25G
Needle Length (b)
¾”
Needle Outer Diameter (c)
0.6 mm
0.5 mm
Needle Length (d)
19 mm
Wall Thickness
Thin Wall (TW)
Tube Length (
l)
35 cm
Tube Length (l)
13.8”
Dead Space Volume (V)
≤ 0.20 ml
Filter Pore Size
20 µm
a x b
c x d
TW
INTENDED PURPOSE
The Suro™ Winged Infusion Set with Filter and Needle
Protection (Surshield™) is intended to access the peripheral
vascular system, for single-dose or short-term intravenous
administration of uids using a syringe or another compatible/
appropriate device. Additionally, after withdrawal of the needle
from the patient’s vein, the safety shield (needle protection
(Surshield™)) shall be manually activated to cover the needle to
minimize risk of accidental needle stick.
INDICATIONS
The Suro™ Winged Infusion Set with Filter and Needle
Protection (Surshield™) is for general application - for
treatment (administration of uids).
CLINICAL BENEFIT
The Suro™ Winged Infusion Set with Filter and Needle
Protection (Surshield™) has an indirect clinical benet (indirect
PATIENT TARGET GROUP
Intended for general application.
INTENDED USERS
Healthcare professional or lay person.
CONTRA-INDICATIONS
No contra-indications.
WARNINGS
• Do not use if unit package is damaged.
• Use immediately after opening the unit package.
• The needle is made of stainless steel containing nickel and
cobalt. Cobalt is classied as CMR* 1B and is present in a
concentration above 0.1% weight by weight. Current scientic
evidence supports that medical devices manufactured from
stainless steel alloys containing cobalt do not cause an
increased risk of cancer or adverse reproductive eects.
* CMR = Carcinogenic, mutagenic or toxic to reproduction (CLP
Regulation EU 1272/2008)
PRECAUTIONS
• Do not use for blood collection.
• After use, secure the needle into the safety shield according to
the directions for use.
• If the needle is bent or damaged, no attempt should be made
to straighten the needle or use the product.
• After use, dispose of safely as medical waste in a sharps disposal
container and/or according to health institution policies. The
product is biohazardous and is physically hazardous due to its
sharp edge.
l cm
l = 13.8" / 35 cm
V ≤ 0.20 ml
Non toxic
medical eect) since it is used for administration of uids.
For general application. The safety shield (needle protection
(Surshield™)) has an indirect clinical benet since it prevents
needle stick injury.
 Loading...
Loading...