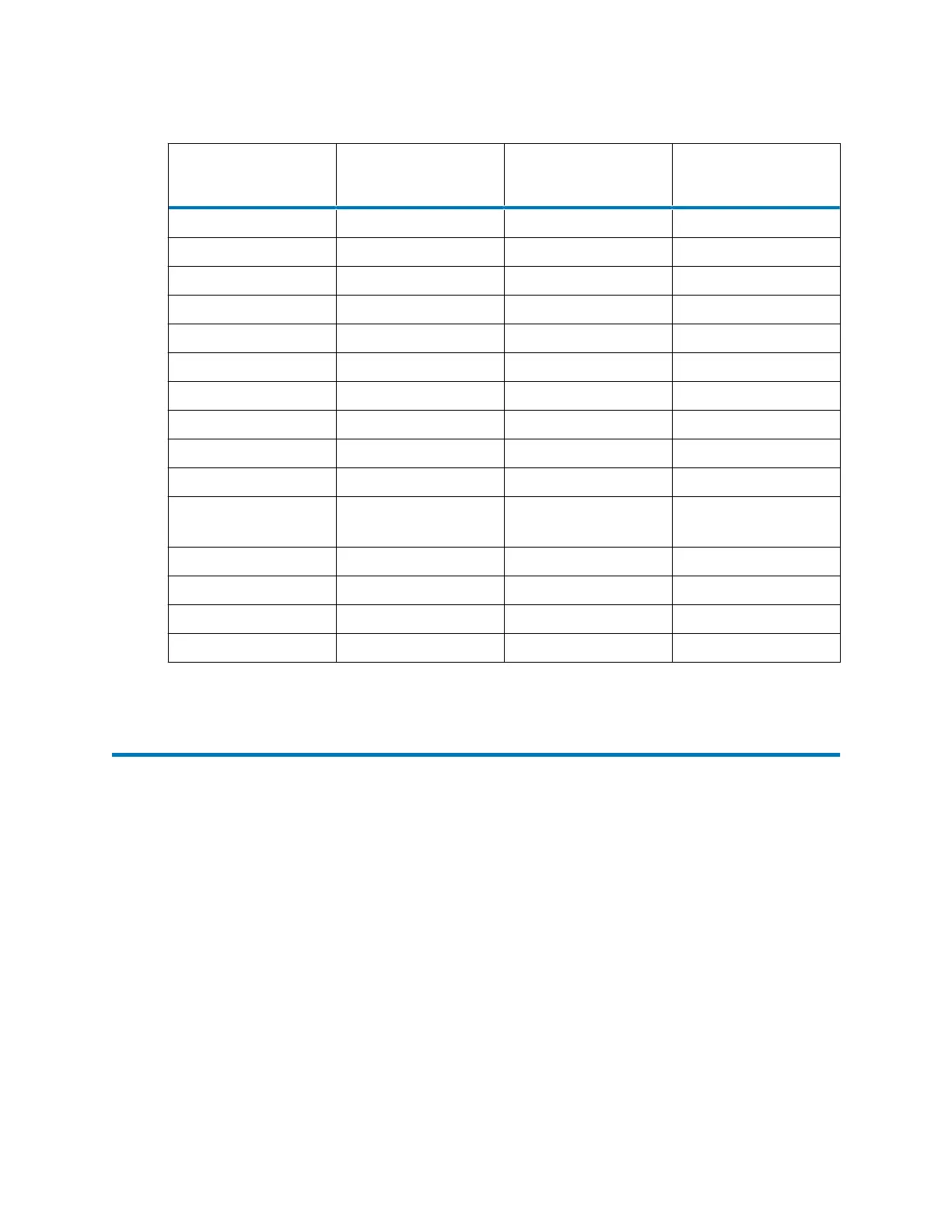
Table D–1: Properties of common solvents (continued)
Solvent Vapor pressure mm
Hg (Torr)
Boiling point (°C) Flash point (°C)
Methanol 97 at 20 °C 64.7 11
Methyl t-butyl ether 240 at 20 °C 55.2 -28
Methyl ethyl ketone 74 at 20 °C 79.64 -9
Methyl isobutyl ketone 16 at 20 °C 117.4 18
N-Methylpyrrolidone 0.33 at 25 °C 202.0 86
Pentane 420 at 20 °C 36.07 -49
n-Propyl alcohol 15 at 20 °C 97.2 23
Propylene carbonate 0.13 at 20 °C 241.7 135
Pyridine 18 at 25 °C 115.25 20
Toluene 28.5 at 20 °C 110.62 4
1,2,4-
Trichlorobenzene
1 at 20 °C 213.5 106
Triethylamine 57 at 25 °C 89.5 -9
Trifluoroacetic acid 97.5 at 20 °C 71.8 -3
Water 17.54 at 20 °C 100.0
o-xylene 6 at 20 °C 144.41 17
D.5 Solvent miscibility effects
Before you change solvents, refer to the following table to determine solvent miscibility. Be aware
of these effects:
• Changes involving two miscible solvents are made directly. Changes involving two solvents
that are not totally miscible (for example, from chloroform to water) require an intermediate
solvent like n-propanol.
• When you switch from a strong buffer to an organic solvent, thoroughly flush the system using
water before you add the organic solvent (see Pure water is required).
• Temperature affects solvent miscibility. If you are running a high-temperature application,
consider the effect of the higher temperature on solvent solubility.
• Buffers dissolved in water can precipitate when mixed with organic solvents.
November 26, 2019, 715003588 Revision C
Page 115
 Loading...
Loading...







