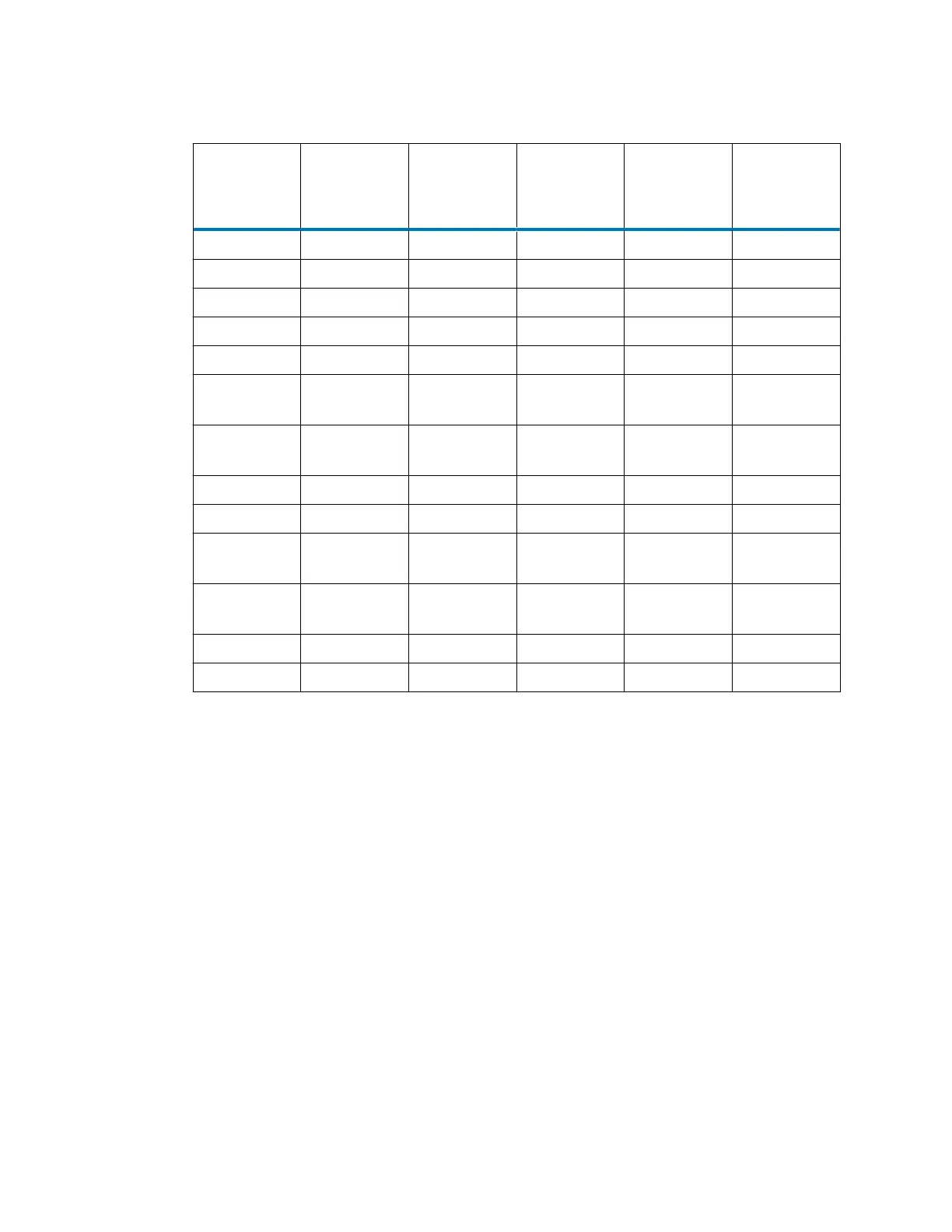
Table D–2: Solvent miscibility
Polarity
index
Solvent Viscosity cP,
20 °C
(@1atm)
Boiling
point °C
(@1atm)
Miscibility
number (M)
λ cutoff (nm)
0.0 N-hexane 0.313 68.7 29 —
1.8 Triethylamine 0.38 89.5 26 —
4.3 1-propanol 2.30 97.2 15 210
4.3 2-propanol 2.35 117.7 15 —
5.2 Ethanol 1.20 78.3 14 210
5.5 Benzyl
alcohol
5.80 205.5 13 —
5.7 Methoxyethan
ol
1.72 124.6 13 —
6.2 Acetonitrile 0.37 81.6 11, 17 190
6.2 Acetic acid 1.26 117.9 14 —
6.4 Dimethylform
amide
0.90 153.0 12 —
6.5 Dimethylsulfo
xide
2.24 189.0 9 —
6.6 Methanol 0.60 64.7 12 210
9.0 Water 1.00 100.0 — —
D.5.1 Using miscibility numbers (M-numbers)
Use miscibility numbers (M-numbers) to predict the miscibility of a liquid with a standard solvent.
To predict the miscibility of two liquids, subtract the smaller M-number value from the larger M-
number value.
• When the difference between the two M-numbers is 15 or less, the two liquids are miscible, in
all proportions, at 15 °C.
• A difference of 16 indicates a critical solution temperature from 25 to 75 °C, with 50 °C as the
optimal temperature.
• When the difference is 17 or greater, the liquids are immiscible, or their critical solution
temperature is above 75 °C.
Some solvents prove immiscible with solvents at both ends of the lipophilicity scale. These
solvents receive a dual M-number:
November 26, 2019, 715003588 Revision C
Page 116
 Loading...
Loading...







