Recommended Qualification Schedule | 21
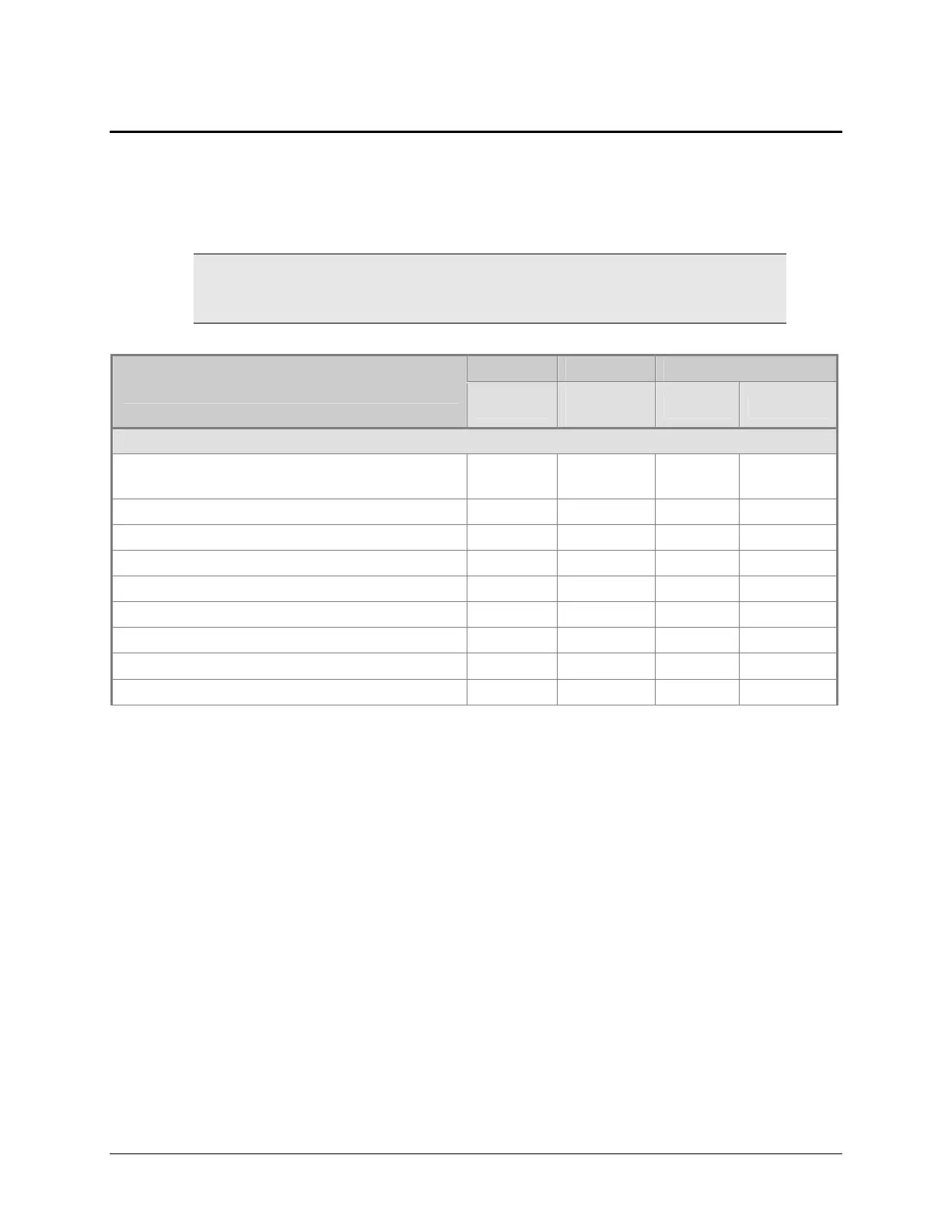
Recommended Qualification Schedule
The schedule shown below defines the factory-recommended intervals for
performance testing for an Epoch used for one shift seven days a week.
The risk factors associated with your tests may require that the
Operational and Performance Qualification procedures be performed
more or less frequently than shown below.
IQ OQ PQ
Tasks/Tests
Initially
Initially/
Annually
Monthly Quarterly
All models:
Unpacking, installation, setup, and
verification
9
Software Documentation/Verification
9 9 9
Software Wavelengths Table Verification
9 9 9
System Test
9 9 9
“Run Assay” Test
9 9
Absorbance Plate Test
9 9
Absorbance Liquid Test 1
9
9
Absorbance Liquid Test 2*
9
9
Absorbance Liquid Test 3 (optional)**
9
9
* If you have an Absorbance Test Plate, run Liquid Test 1. If you do not have an Absorbance
Test Plate, run Liquid Test 2.
** Liquid Test 3 is optional; it is provided for sites requiring verification at wavelengths lower
than those attainable with the Absorbance Test Plate.
Epoch Operator’s Manual
 Loading...
Loading...