Metrological certificate of approval
106
Information on DrägerSensor O
2
(6809720)
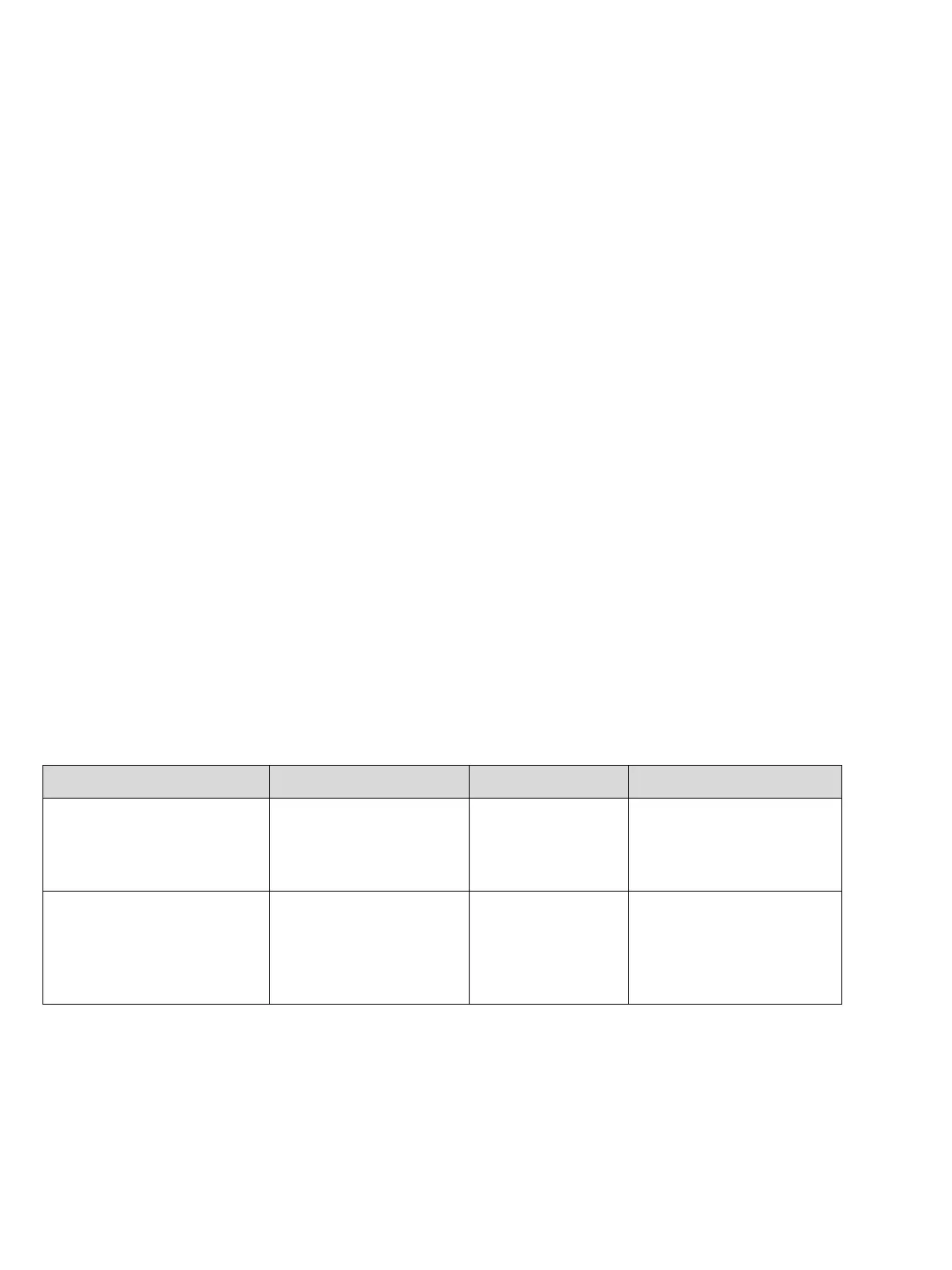
Cross sensitivities
No cross sensitivities against pollution gases with a range up to 100 ppm. For gases in a concentration range larger than 1 Vol.-
%: see table. The influence of the O
2
displacement is in not taken into account in the table (partial pressure measurement).
Increased sensor drift can be caused by high sour gas concentrations (> 1 Vol.-%), which could make shorter calibration inter-
vals necessary. The sensor service life is reduced in dependence on the duration and the concentration of the sour gases (e.g.
lifetime in atmospheres containing CO
2
: 5000 Vol.-% CO
2
x hours).
Organic solvents (e.g. acetone, propyl alcohol, etc.) dissolve in the plastic parts of the sensor. If allowed to react over several
days in larger concentrations (> 1 Vol.-%), these substances can cause sensor drift can which could make shorter calibration
intervals necessary. This will not reduce the sensor service life.
Principle of measurement: The DrägerSensor O
2
(6809720) is an electrochemical two-electrode-sensor for measurement of oxygen (O
2
)
in air. The sensor can only be operated in connection with a suitable Dräger transmitter.
Reaction at the measuring electrode: O
2
+ 2 H
2
O + 4 e
–
=> 4 OH
–
Reaction at the counter-electrode: 2 Pb => 2 Pb
2
+ + 4 e
–
Ambient conditions:
Operational Characteristics
–5
o
C to 40
o
C, temporarily –20
o
C to 55
o
C
700 hPa to 1300 hPa
10 to 95 % relative humidity, non condensing
Storage 0
o
C to 40
o
C
700 hPa to 1300 hPa
30 to 70 % relative humidity, non condensing
Measured value time setting:
t
0...20
<12 seconds
t
0...90
<30 seconds
Note: In the case of temperatures below –5
o
C, the measured value time setting may increase.
Calibration:
Flow rate: 0.5 L/min
Zero gas Nitrogen (99.9 Vol.-% N
2
)
Calibration gas Oxygen / nitrogen – mixed gas
Warming-up time:
Sensor is ready to operate after 15 minutes
Sensor is ready to calibrate after 2 hours
Gas / vapour Chemical symbol Gas concentration Measurement value deviation
in Vol.-% O
2
acetone CH
3
COCH
3
1 Vol.-% 0.1
ethane C
2
H
6
10 Vol.-% 0.1
ethanol C
2
H
5
OH 1 Vol.-% 0.1
ethylene C
2
H
4
5 Vol.-% 0.1
ethine C
2
H
2
2 Vol.-% 0.1
carbon dioxide CO
2
5 Vol.-% 0.1
carbon monoxide CO 1 Vol.-% 0.1
methane CH
4
10 Vol.-% 0.1
methanol CH
3
OH 1 Vol.-% 0.1
propane C
3
H
8
5 Vol.-% 0.1
hydrogen H
2
10 Vol.-% 0.1
 Loading...
Loading...