9.3 Calibrating total chlorine
9.3.1 Purpose
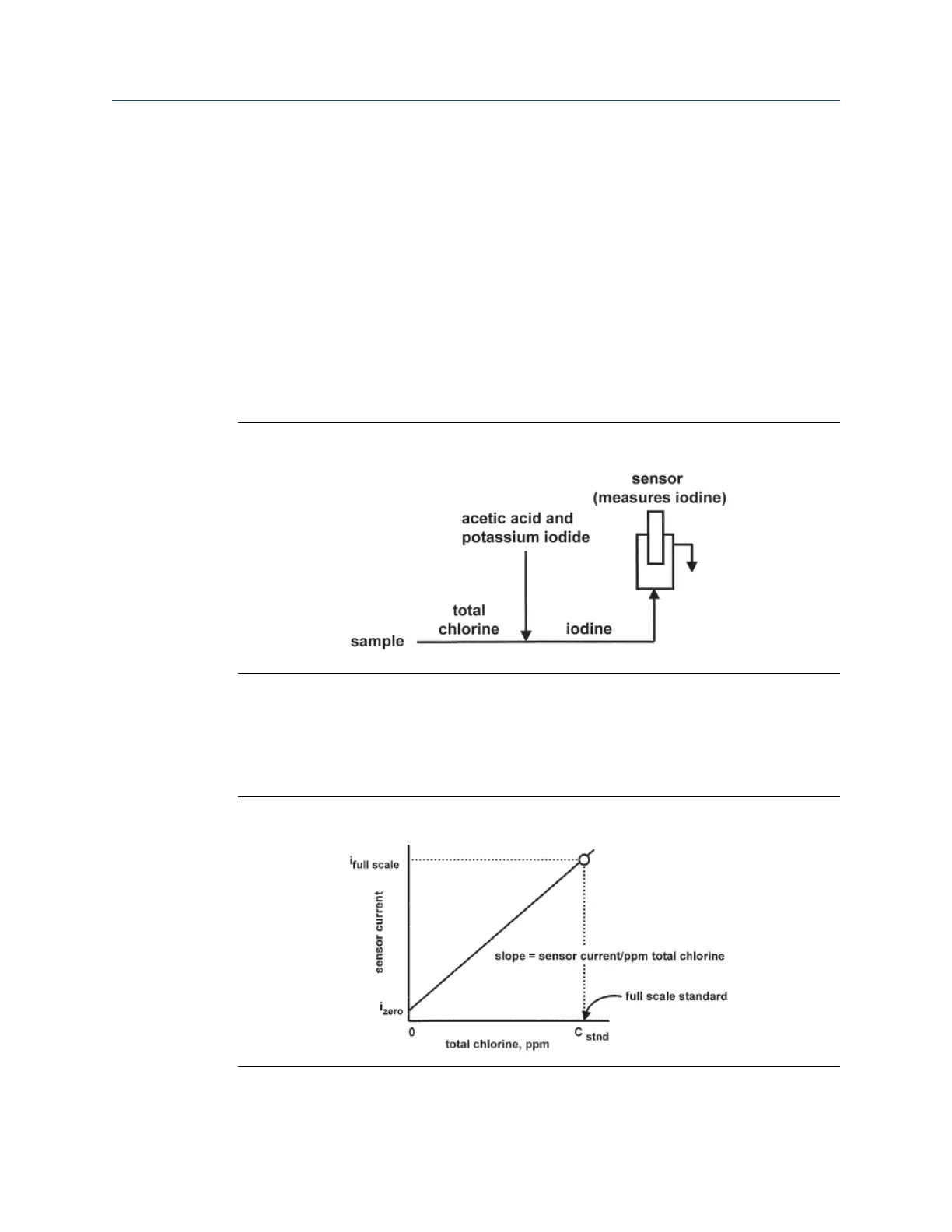
The continuous determination of total chlorine requires two steps. See Figure 9-1. First, the
sample flows into a conditioning system (the Rosemount TCL) where it is treated with
acetic acid (vinegar) and potassium iodide. The acid lowers the pH, which allows total
chlorine in the sample to quantitatively oxidize the iodide to iodine. The treated sample
then flows to the sensor. The sensor is a membrane-covered amperometric sensor, whose
output is proportional to the concentration of iodine. Because the concentration of iodine
is also proportional to the concentration of total chlorine, the transmitter can be calibrated
to read total chlorine.
Determination of Total ChlorineFigure 9-1:
Figure 9-2 shows a typical calibration curve for a total chlorine sensor. Because the sensor
really measures iodine, calibrating the sensor requires exposing it to a solution containing
no iodine (zero standard) and to a solution containing a known amount of iodine (full-scale
standard).
Sensor Current as a Function of Total Chlorine ConcentrationFigure 9-2:
Calibration
Instruction Manual 63

 Loading...
Loading...











