10
9
8
7
6
5
4
3
2
1
Appendix
Reference
Guides
Alarms and
Emergencies
Patient
Management
Surgical
Implant and
Explant
Monitor
Peripherals
and
Accessories
HVAD
®
PumpOverviewIntroduction
13Introduction
1.7 Pivotal US Clinical Study: Bridge-to-Transplant (continued)
Table 2: Success Rates and Inference on Non-Inferiority
Implanted
Successes
UCL (%) p-value
Safety Cohort
HVAD
®
140 127 (90.7)
4.5 <0.0001
Controls 497 448 (90.1)
Per Protocol Cohort
HVAD
®
137 126 (92.0)
0.9 <0.0001
Controls 497 448 (90.1)
consent before 180 days, have a missing success/failure outcome.
Competing Outcomes
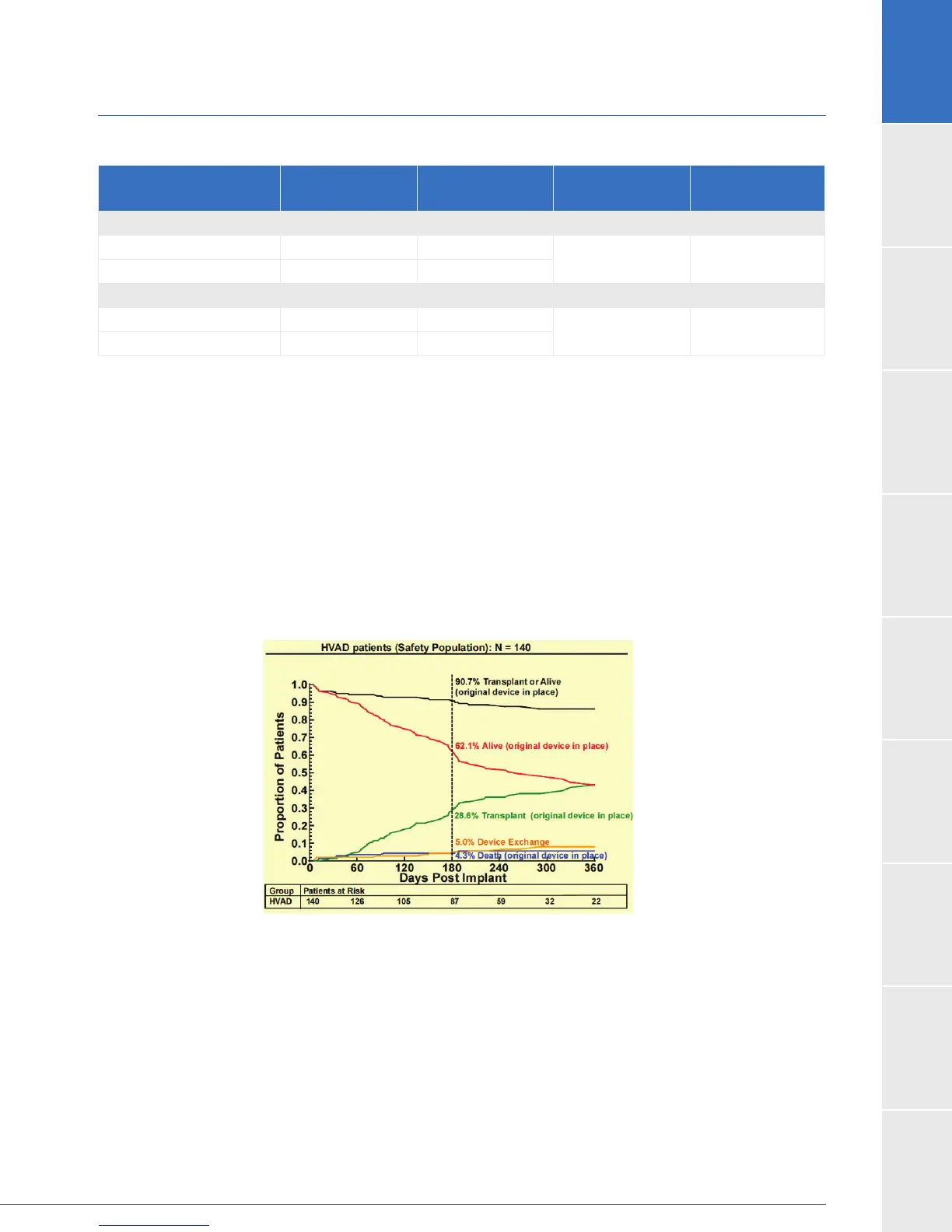
A competing risks analysis was performed (Figure 2), estimating the time-related probability
of experiencing each of the component events. These data are calculated from all events
occurring during the study duration, including deaths, transplants and exchanges occurring
after 180 days but ending with last-patient, last-visit.
Figure 2: Competing Risk Outcomes (HVAD Safety Population)
Deaths
There were eight subject deaths during the 180-day study period. Six deaths occurred in subjects
with their originally implanted device and two deaths occurred after device exchange.
Safety Results
This study was not randomized and used a contemporaneous control for the sole purpose of
HeartWare
™
HVAD
™
System and have no randomized comparator arm.
 Loading...
Loading...